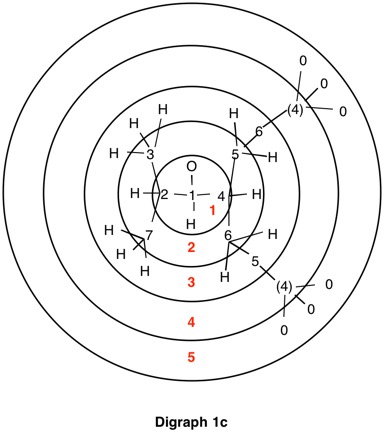
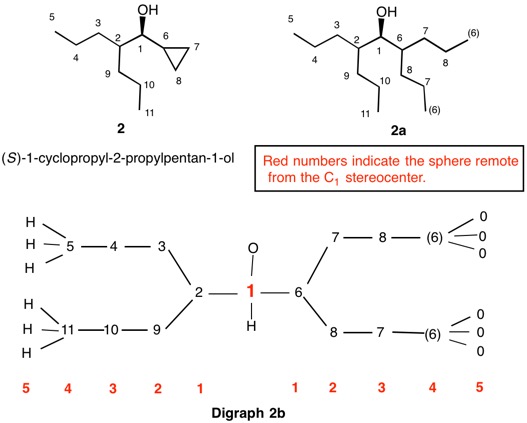
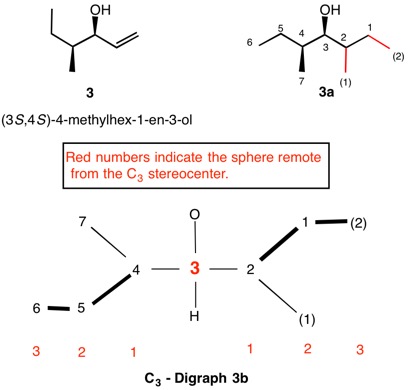
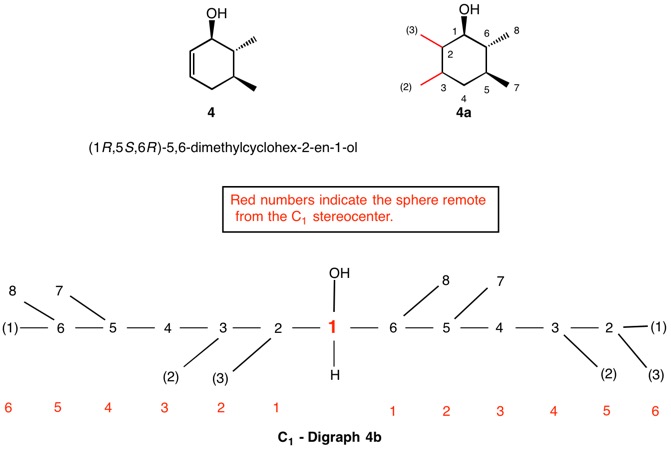
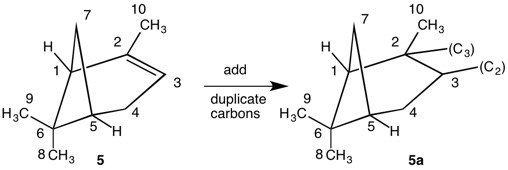
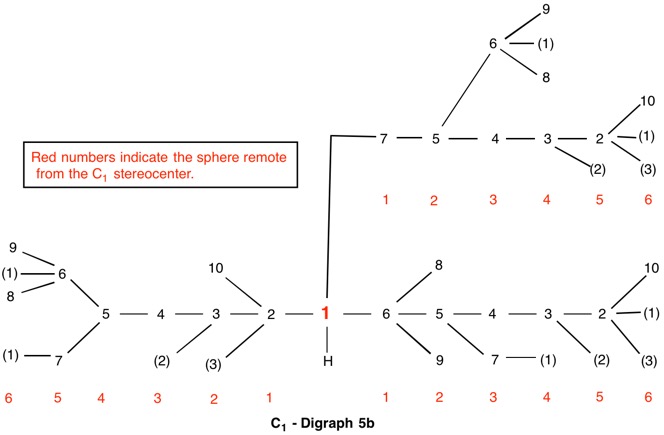
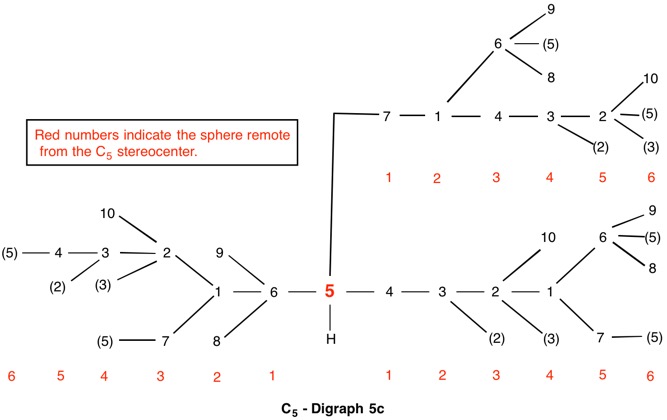
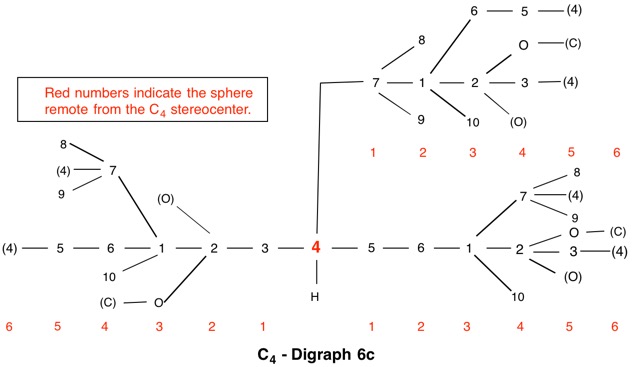
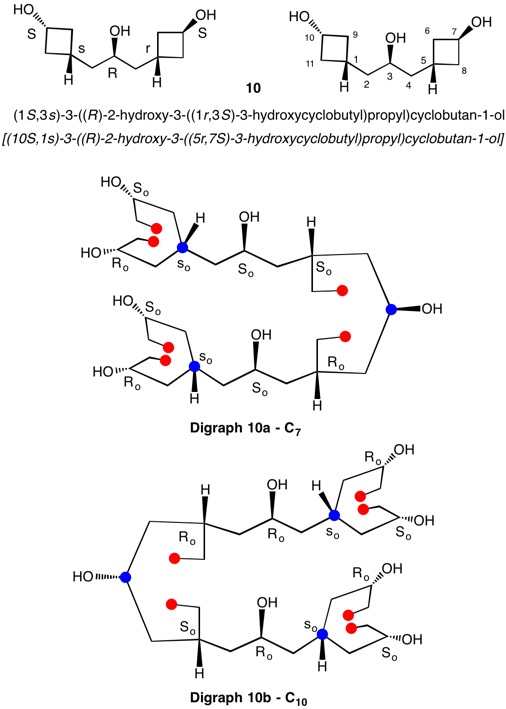
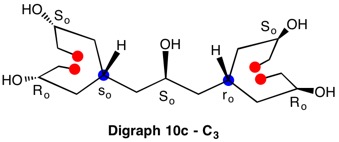
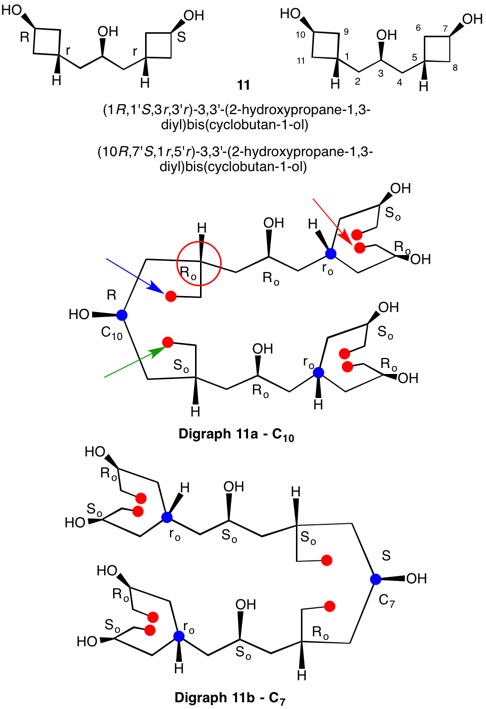
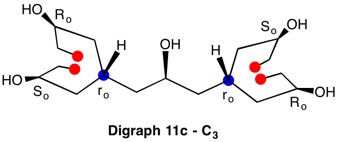
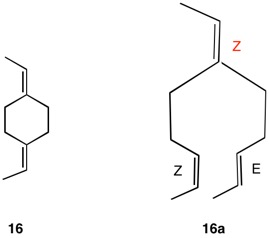
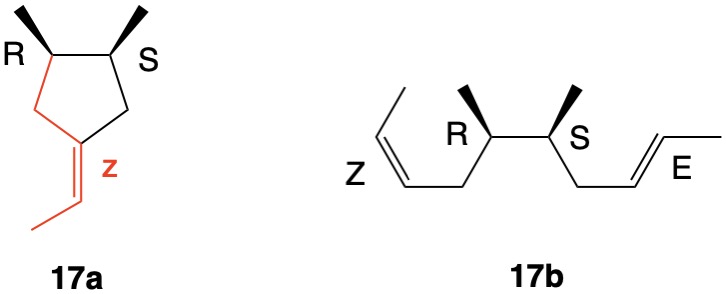
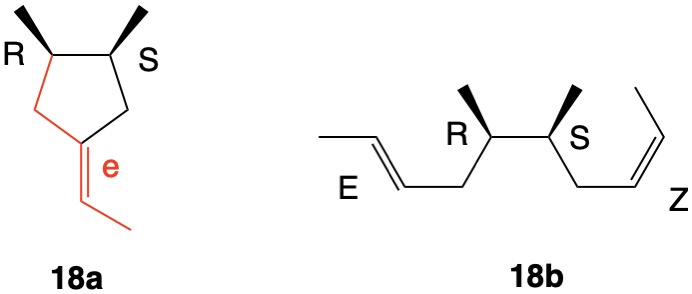
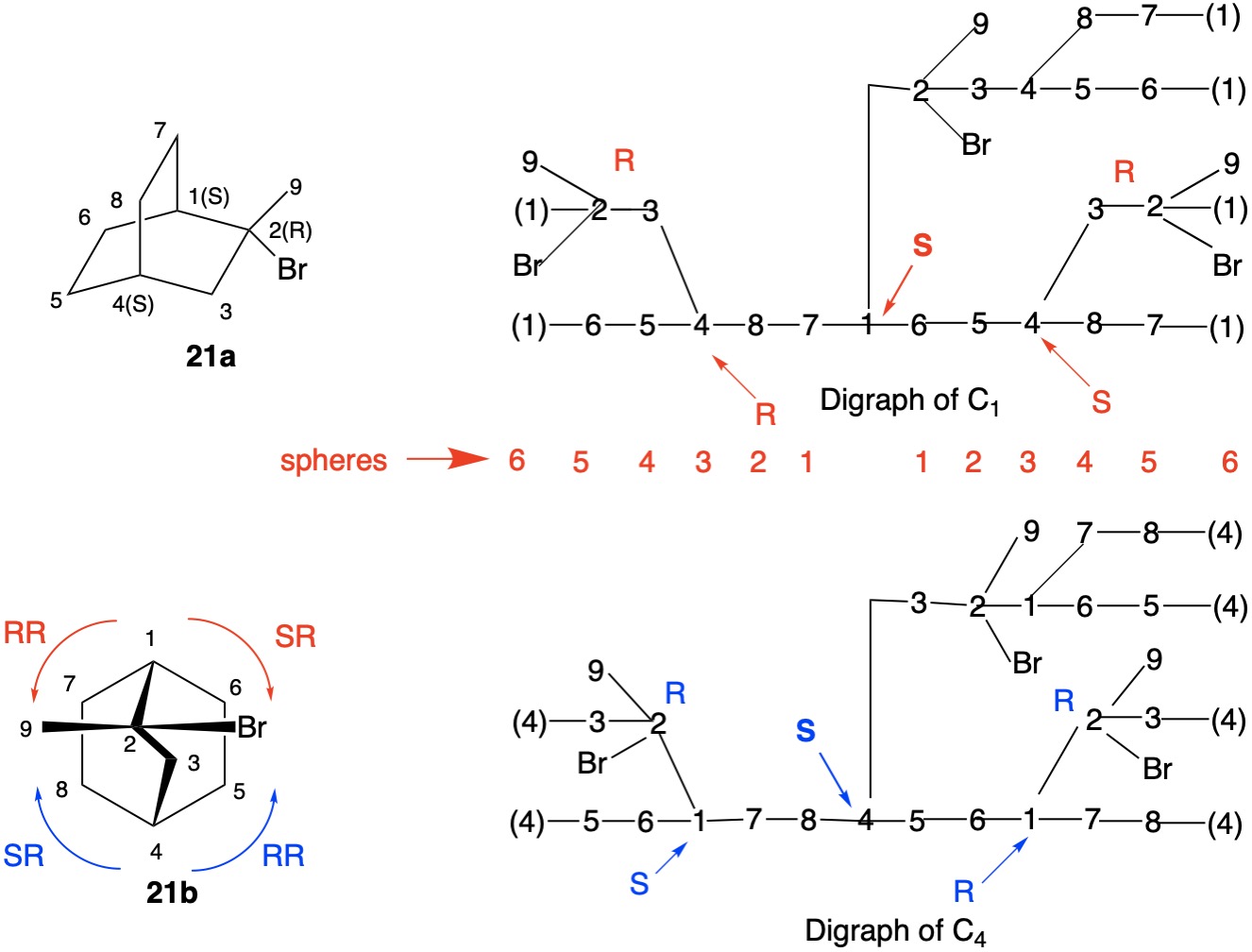
The assignment of R/S-configurations to the enantiomers of 2-butanol is trivial by the CIP method. But how does one handle rings as substituents? The solution to the problem is to convert the structure to an acyclic version, a digraph as in 1a and 1b.
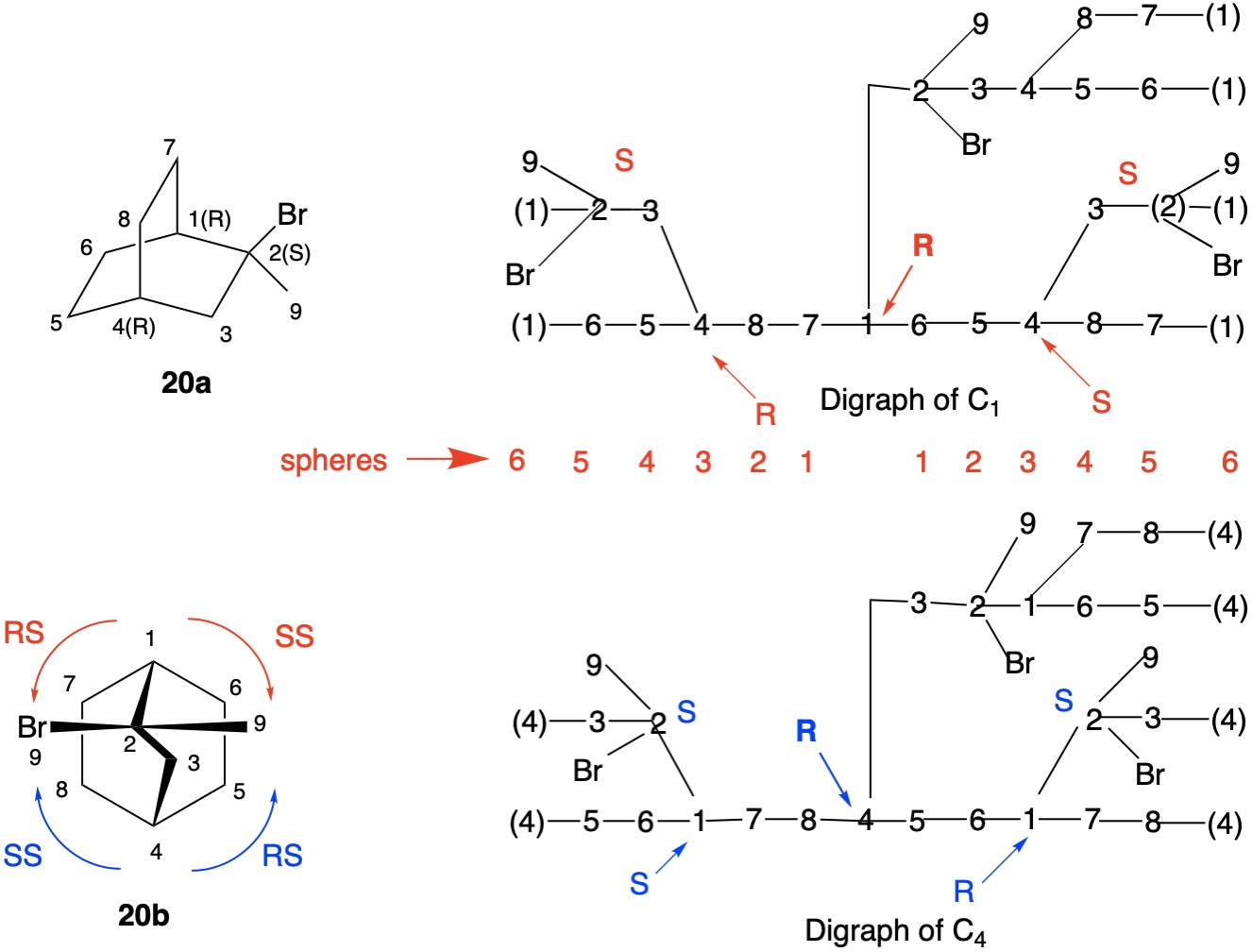
Using cyclopropyl alcohol 1 as an example, consider the path C4-C5-C6-C4 around the ring. The path C4-C5-C6-(C4), wherein the last term in the chain is a tetradentate, duplicate carbon attached to three atoms of atomic number zero (phantom atoms). The duplicate atoms, in this instance carbon, are enclosed in parentheses and still have their respective atomic number. The phantom atom ranks below hydrogen because they have atomic number zero.
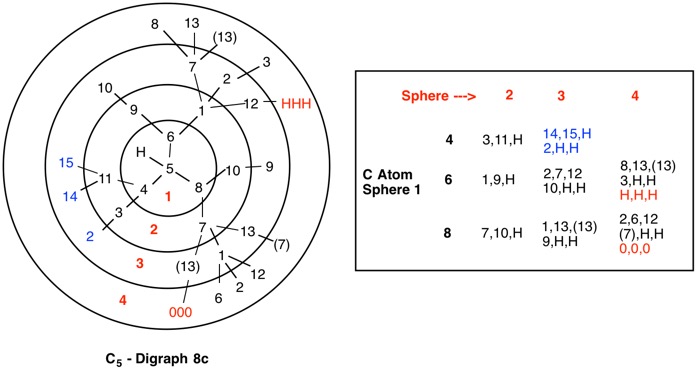
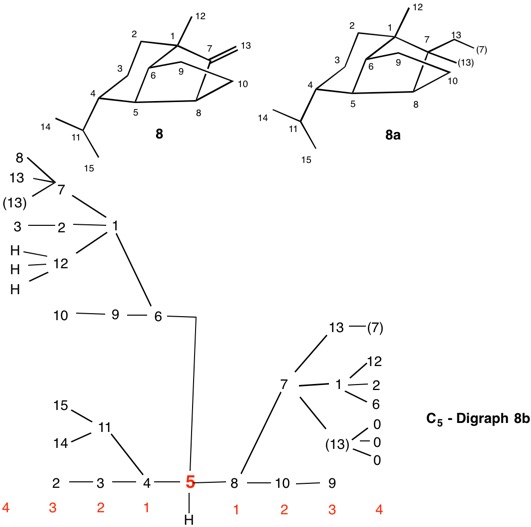
In digraphs 1a and 1b the number of the duplicate atom is displayed as (4). Alternatively, the path around the ring could also be traversed via route C4-C6-C5-(C4).
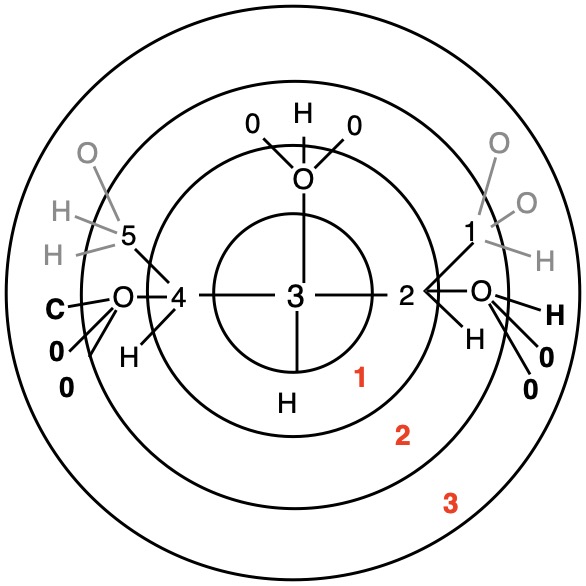
The red numbers in digraph 1b or 1c signify the sphere or the distance the atoms are remote from the center of stereochemistry. The oxygen, C2, C4 and H are in the first sphere with oxygen having the top priority and the hydrogen the lowest. The priority of the C2 and C4 chains must be determined. No priority can be assigned at sphere 1 because O>C2= C4>H. C2 and C4 are equivalent [C,C,H] in sphere 2. Sphere 3 breaks the tie where C4 [C,H,H] supercedes C2[H,H,H]. The priorities for C1 are O>C4>C2>H. The stereocenter is of the (R)-configuration.
Notice that spheres 4 and 5 were not utilized. Sphere 4 contains a duplicate atom (4) while sphere 5 contains six phantom atoms of at. no. = 0. Most of the subsequent digraphs will not display hydrogens or phantom atoms but their presence will be implied.