The Degree, or Elements, of Unsaturation tells you how may rings and multiple bonds are present in a compound if you know the molecular formula. Most textbooks give you a formula to determine the Degree of Unsaturation. Here is an empirical method to do the same thing.
A hydrocarbon of n carbons cannot contain more than 2n+2 hydrogens. CnH2n+2 is the formula for any alkane that is acyclic (not a ring). This statement is true for both normal (straight chain) and branched alkanes. After all, both n-butane and isobutane are isomers (same composition, different structures) C4H10.
Convince yourself that each of the following hydrocarbons fits the formula CnH2n+2.
![]()
When a hydrocarbon has the formula CnH2n, it is two hydrogen atoms shy of being completely saturated. Its degree of unsaturation is 1, which is determined by subtracting H2n from H2n+2 and dividing the difference by 2. A hydrocarbon with a degree of unsaturation of 1 is either an acyclic (non-cyclic) alkene or a cycloalkane. You can imagine taking n-pentane (above left) and mentally removing a hydrogen atom from C1 and C2. What you have left, in addition to H2, is 1-pentene. If the same mental exercise were conducted at C1 and C5 of n-pentane and the two radicals were joined, a cyclopentane ring would be formed.
Convince yourself that each of the following hydrocarbons fits the formula CnH2n..
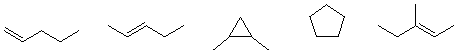
If a compound fits the formula CnH2n-2 it has two degrees of unsaturation. Do the math. This compound may be a triple bond (alkyne), two double bonds (diene), a double bond and a ring (cycloalkene), or two ring (bicyclic alkane).
Convince yourself that each of the following hydrocarbons fits the formula CnH2n.-2.
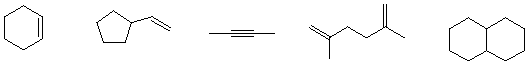
The degree of unsaturation may also be employed in another way. Given a particular hydrocarbon structure for which you know the number of carbons and the degree of unsaturation, you can calculate the number of hydrogen atoms. Benzene has 6 carbon atoms and 4 degrees of unsaturation (1 ring and 3 double bonds). If you work backwards and double the degrees of unsaturation you have 8.
The maximum number of hydrogens for a C6 compound is 14. The difference between 14 and 8 is 6. Benzene is C6H6.
Hydrocarbons must have an even number of hydrogen atoms. If the number of hydrogens is odd, you have a carbocation, carbanion or radical.
Any hydrocarbon, independent of its degree of unsaturation, can be converted into a monohalide by removing a hydrogen atom from the hydrocarbon and replacing it with a halogen. Therefore, the monohalide will have an odd number of hydrogens. A dihalide will have an even number of hydrogens, etc. Remember, this rule is being applied only to alkyl halides. To determine the degree of unsaturation of a halide, remove the halogen from the formula and replace it with hydrogen. Do the computation as though the compound were the new hydrocarbon. For example, C5 H7Cl becomes C5 H8 which has two degrees of unsaturation.
Try the following:

n-Butane has no degrees of unsaturation. If we mentally insert an oxygen atom into a C-H bond we will have an alcohol, either 1-butanol or 2-butanol. Both compounds have zero degrees of unsaturation (no multiple bonds or rings). If the oxygen atom were inserted in a C-C bond of n-butane, ethers would be formed, both of which, diethyl etherand methyl n-propyl ether, have zero degrees of unsaturation. Thus,ignore oxygen atoms in a formula when calculating a degree of unsaturation. Since sulfur is also divalent, ignore it as well.
To convert a nitrogen containing compound to a hydrocarbon, remove the N from the formula and replace it with one C and one H. Thus, pyridine, C5H5N, becomes C6H6, which has four degrees of unsaturation. because nitrogen in its several oxidation states is odd, a CHN compound with an odd number of nitrogen atoms must have an odd number of hydrogens. Since halogens have an odd valence, they can be considered along with nitrogen. Compound C5H4NCl has an even number of hydrogens.
Try the following:

How do you handle formulas with all five atoms? Consider the formula C8H10BrClN2O3. First we see that there are 2 halogen atoms and 2 nitrogens. The number of hydrogens is even. By ignoring the oxygen atoms we have C8H10BrClN2. Substituting 2 hydrogens for the halogens the formula becomes C8H12N2. Finally, The addition of 2 carbons and 2 hydrogens in place of the 2 nitrogens gives C10H14. The most saturated C10 hydrocarbon is C10H22. Thus the degree of unsaturation of C8H10BrClN2O3 is (22-14)/2 = 4. The following compounds all fit the data. Draw some more structures with this formula.
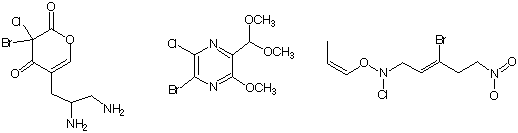
Can the compound C4H11Cl2NO exist? Based upon the presence of 2 chlorines and 1 nitrogen, the odd number of hydrogen atoms is also odd. So far; so good. The formula reduces as follows: C4H11Cl2NO ---> C4H11Cl2N ---> C4H13N ---> C5H14. This structure is more saturated than C5H12. The formula is incorrect.
Although using a formula is an acceptable solution for determining the degree of unsaturation, you should know how it is derived to make it your own. The formula may be derived using the empirical method discussed above. Consider a compound with the molecular formula C8H10NOX where X equals halogen. Using the above method, drop the oxygen and replace the halogen with one hydrogen. This leaves C8H11N. Replace nitrogen with one carbon and one hydrogen. Now we have C9H12. The most saturated nine carbon hydrocarbon is C9H20. Thus, the degree of unsaturation (D.U.) = ( C9H20 - C9H12)/2 = (20 - 12)/2 = 4.
Now, let c = #C, h = #H, n = #N and x = #X (X = halogen)
Any formula containing these elements, ignoring divalent atoms, may be expressed as cC + hH + nN + xX
Removing X and replacing it with H gives cC + hH + nN + xH or cC + (h + x)H + nN
Removing N and replacing it with one C and one H gives cC + (h + x)H + nC + nH or (c + n)C + (h + x + n)H (equation 1)
The most saturated hydrocarbon CmH2m + 2 is (c + n)C + [2(c + n) +2] H (equation 2)
Subtracting equation 1 from equation 2 affords
{(c + n)C + [2(c + n) +2]H} - [ (c + n)C + (h + x + n)H] or [2(c + n) + 2]H - (h + x + n)H
Dropping "H" gives
2c + 2n + 2 - h - x - n or 2c + n - h - x + 2 (equation 3)
Dividing equation 3 by 2 yields
D.U. = (2c + n - h - x + 2)/2 or c + 1 + (n - h - x)/2
Testing the first equation above with the original formula C8H10NOX gives the correct result
D. U. = (16 + 1 - 10 - 1 + 2)/2 = 4
Try the following: C10H12N2O3X2
D. U. = (20 + 2 - 12 - 2 + 2)/2 = 5
Once again, remember that in compounds having all valences utilized and the sum of odd valenced atoms is even, then the number of hydrogens must be even. Likewise, if the sum of odd valenced atoms is odd, the number of hydrogens must be odd. If not, one is dealing with a carbocation, radical or anion. The D. U. will not be a whole number. Consider C10H11N2O3X2: D. U. = (20 + 2 -11 -2 +2)/2 = 5.5.
If the formula has too many hydrogens as in C10H26N2O3X2, the value of the D. U. will be negative.
D. U. = (20 + 2 - 26 - 2 + 2)/2 = - 4.