The R and S Convention
for Absolute Configuration
for Absolute Configuration
The absolute stereochemistry of chiral molecules is defined by the R/S convention developed in 1956 by R. S. Cahn, C. Ingold and V. Prelog. R stands for righthanded (L., rectus: right, in the sense of being correct) and S for lefthanded (L., sinister: improper).
[One might question why the Latin for "on the right", dexter, and "on the left", laevus (levus) were not adopted. Unfortunately, "d" and "l" were already in use for the direction of rotation of plane polarized light by optical active compounds (enantiomers). "D" and "L" were already in use to describe the absolute configuration of molecules by their synthetic relationship to the D- and L-configurations of glyceraldehyde. This latter method is not a fool proof system because two different enantiomers of a compound can sometimes be prepared from the same enantiomer of glyceraldehyde. One wag has suggested that Cahn wished his initials to be the descriptors.]
Only the rules for compounds containing an asymmetrically substituted atom will be considered.
Atoms attached directly to an asymmetrically substituted quadrivalent atom (it need not be carbon) are assigned their respective atomic numbers (not masses). They are ranked in descending order with the highest value assigned 1 and the lowest 4.
Rule 2:
When ties are encountered, the substituents on these atoms are used to break the tie between the atoms. The priorities of Rule 2 do not supercede the priorities in Rule 1. When isotopes are encountered, the heavier mass has the higher priority.
Rule 3:
Treat multiple bonds as though they were bonded to single atoms of the same type. A C=C bond becomes C-C-C-C and a carbonyl group (C=O) is treated as O-C-O-C. The atoms in red are classified as "ghost atoms".
Examples:
Rule 1:
There are two general methods for determining the R- or S-configuration: The hand method and the clock method. The hand method points the thumb in the direction of the atom of lowest priority. The curled fingers point in the direction of descending priority of the remaining atoms. If the curled fingers don't do so, the chirality is of the opposite handedness. Test this technique on either structure in first illustration below, (R)-bromochloroiodomethane. The chirality is R in both cases. The structures are superimposable by translation and rotation through space. The clock method requires the viewer to rotate the structure so that the lowest priority atom is remote to the viewer. If the descending order of the remaining three atoms proceeds in a clockwise fashion -- as they do in the right hand structure of bromochloroiodomethane -- then the chirality is R. Counterclockwise rotation is the S-configuration. Imagine the arrows in the right hand structure as a wire threaded through the four atoms attached to carbon. The wire describes a spiral -- a chiral object.The advantage of the hand method over the clock method is that with the former method the structure does not have to be moved or rewritten, thus eliminating the opportunity for an error in transcription. Click the "spin off" button on the JSmol enantiomers and move them with your touchpad or mouse to match the static structure to their right. Next, move the JSmol structures so that they mirror one another.
(R)-Bromochloroiodomethane
How to manipulate JSmol structures
Descending priority: I
---> Br ---> Cl ---> H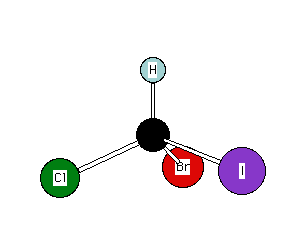
Using the structure on the left, your thumb points to the
atom of lowest priority (H, 4).

Your fingertips point in the descending order I,(1),
Br(2),Cl(3). The right hand indicates the R configuration.
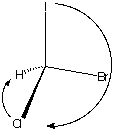
Rotate the structure on the left so that the lowest
priority atom is in the rear. I ---> Br ---> Cl
---> H proceeds in a clockwise direction. The
configuration is R.
(R)-Bromochloroiodomethane
|
How to manipulate JSmol structures |
Descending priority: I
---> Br ---> Cl ---> H  |
Using the structure on the left, your thumb points to the atom of lowest priority (H, 4).
Your fingertips point in the descending order I,(1), Br(2),Cl(3). The right hand indicates the R configuration. |
Rotate the structure on the left so that the lowest priority atom is in the rear. I ---> Br ---> Cl ---> H proceeds in a clockwise direction. The configuration is R.
|
|---|
(S)-Bromochloroiodomethane
How to manipulate JSmol structures
Descending priority: I ---> Br
---> Cl ---> H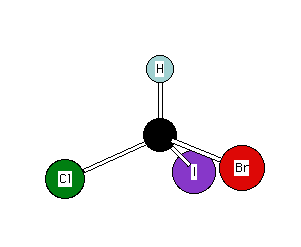
Descending priority: I ---> Br
---> Cl ---> H
Using the structure on the left, your thumb points to the
atom of lowest priority (H, 4)
Your fingertips point in the descending order I,(1),
Br(2),Cl(3). The left hand indicates the S configuration.
Rotate the structure on the left so that the lowest
priority atom is in the rear. I ---> Br ---> Cl
---> H proceeds in a counterclockwise direction. The
configuration is S.
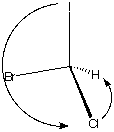
(S)-Bromochloroiodomethane
|
How to manipulate JSmol structures |
Descending priority: I ---> Br
---> Cl ---> H  |
Descending priority: I ---> Br
---> Cl ---> H
Using the structure on the left, your thumb points to the atom of lowest priority (H, 4)
Your fingertips point in the descending order I,(1), Br(2),Cl(3). The left hand indicates the S configuration. |
Rotate the structure on the left so that the lowest priority atom is in the rear. I ---> Br ---> Cl ---> H proceeds in a counterclockwise direction. The configuration is S.
|
|---|
Rule 2:
2-Methylbutan-1-ol (shown below) can exist as a pair of enantiomers. The asymmetrically substituted carbon (red arrow) has four different substituents, three of which are carbon atoms and the remaining one a hydrogen atom. The red level priorities are: 6, 6, ,6, 1. The hydrogen is the lowest priority. One of the three carbons has an oxygen and two hydrogens attached (8,1,1), a second one carbon and two hydrogens attached (6,1,1) and a third has three hydrogens attached (1,1,1). The carbon bearing the oxygen becomes the first priority atom, the carbon bearing a carbon is the second priority atom, and the methyl group is the third priority atom. The first difference (underlined) establishes the priority at the blue tie breaker level. What is the chirality of the second structure?

Test your ability to assign the absolute configuration to each representation of the 2-methylbutan-1-ols, 1-6 using the hand method. Alternatively, use the JSmol enantiomers of 2-methylbutan-1-ol to assist you in matching the structures 1-6. Click here for answers.
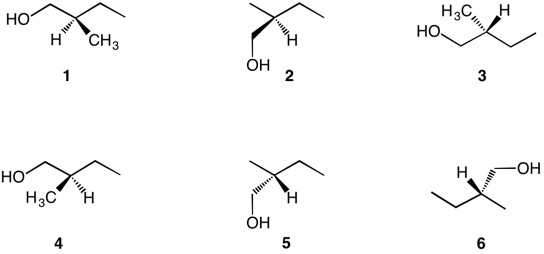 |
(S)-2-Methylbutan-1-ol
How to manipulate JSmol structures |
|---|
Rule 3:
An enantiomer of glyceraldehyde (a.k.a., L-glyceraldehyde) is shown below. When multiple bonds are encountered, such as in the case of the carbonyl group, the double bond is treated as a single bond with a phantom oxygen (in blue) attached to the carbon and a phantom carbon (in blue) attached to the oxygen. After all, in a carbonyl the carbon atom is bound twice to oxygen and the oxygen is bound twice to carbon. The tie breaker in this case is between two carbons, one being bound to two oxygens, the other to one oxygen. L-Glyceraldehyde is of the S-configuration. For interactive exercises, click here.
| L- Glyceraldehyde
How to manipulate JSmol structures |
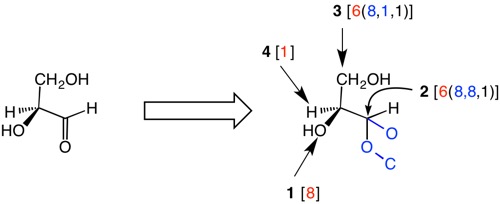 |
|---|