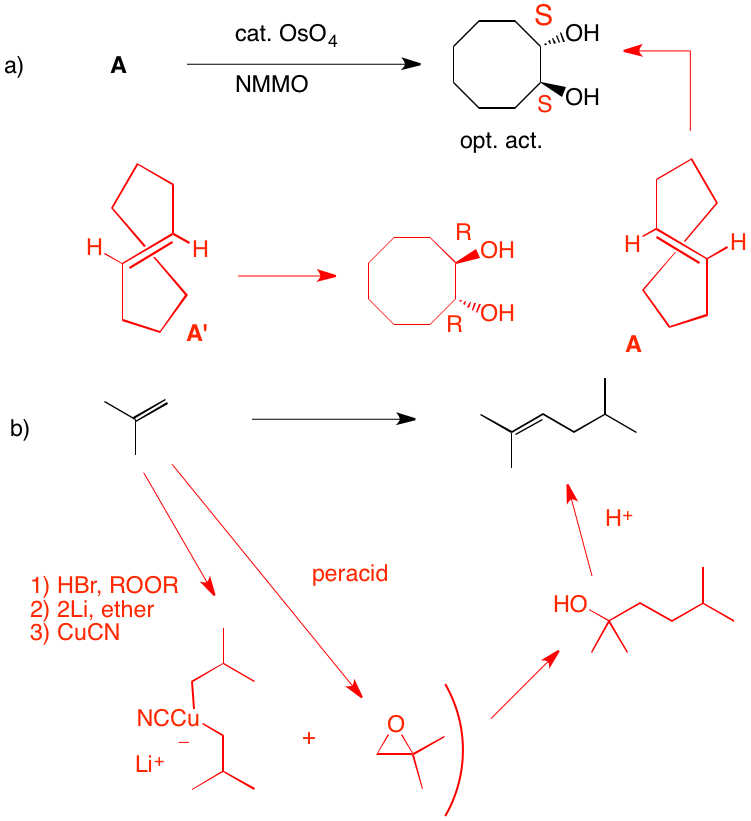
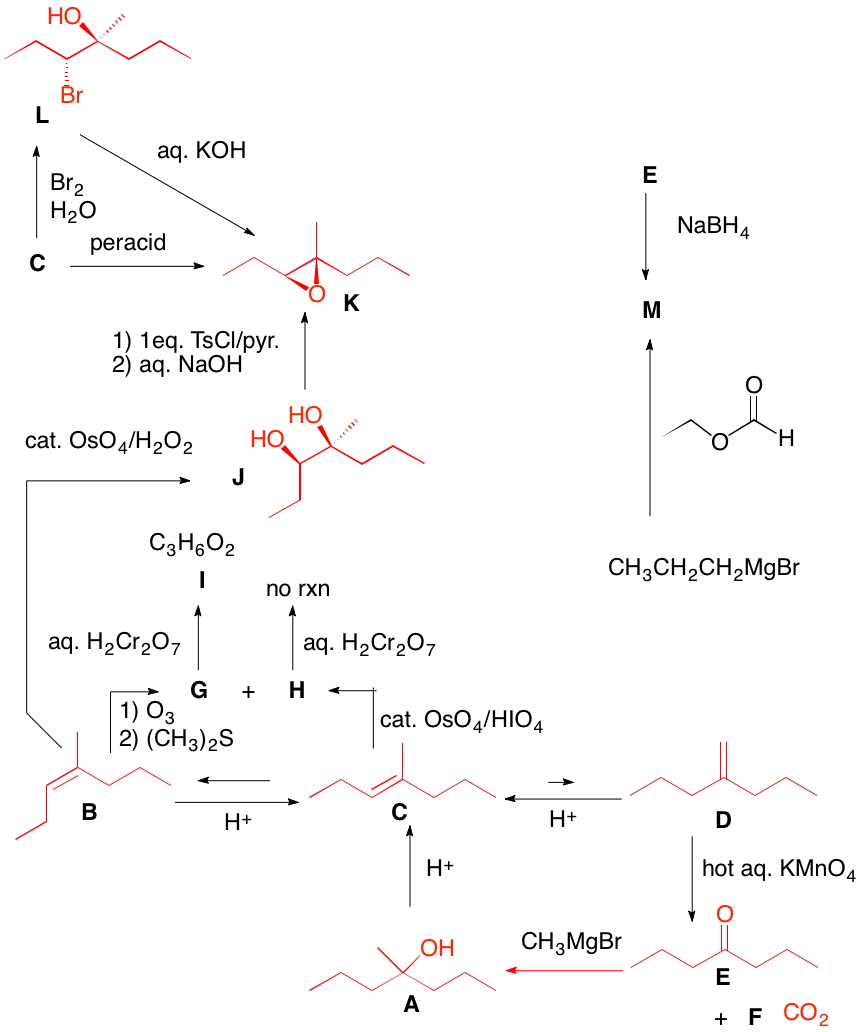
a) The diol is optically active (S, S) and trans. Since OsO4 adds syn to a double bond, A cannot be cis-cyclooctene, which would provide the cis-diol. A is the enantiomer of trans-cyclooctene shown. Its enantiomer, A', would afford the R,R-enantiomer. The reaction of OsO4 with the double bond of trans-cyclooctene can only react with the external face because the internal face is hindered by the methylene chain.
b) Isobutylene must be used twice and a carbon-carbon bond must be formed. The epoxide is the electrophile and the cuprate reagent is the nucleophile. Cuprates may be generated from Grignard reagents (or organolithium reagents). The problem with magnesium cations, and Grignard reactions with highly substituted epoxides, is that they can effect rearrangement of epoxides. In this instance isobutyraldehyde can form and act as an electrophile toward the magnesium reagents. Attack occurs in an SN2 fashion at the less substituted carbon of the epoxide to afford the diol. Acid catalyzed E1 dehydration gives the more substituted double bond.
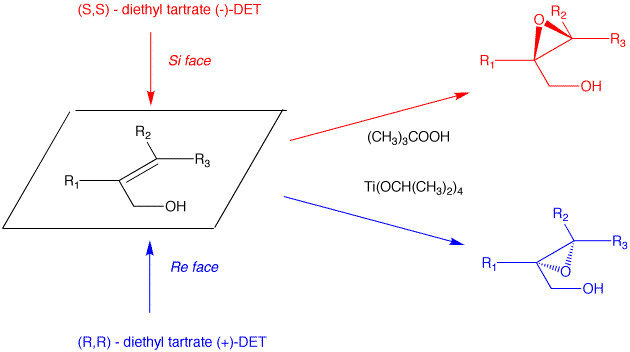
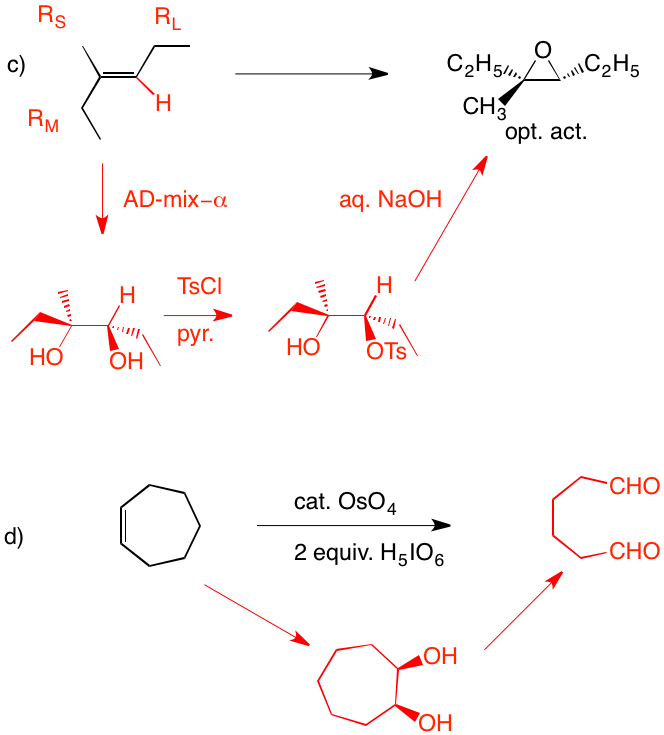
c) Direct epoxidation or halohydrin formation followed by base will give racemic epoxide. Moreover an direct mode of epoxidation will not provide the correct stereochemistry. There must be one inversion of stereochemistry. The epoxide can be formed from the tosylate via internal displacement by the alkoxide of the alcohol. The requisite diol arises by Sharpless asymmetric dihydroxylation. The optically active diol is formed from the achiral alkene with AD-mix-α. [Go here for a review.]
d) cis-1,2-Cycloheptanediol is formed in situ. Cleavage to the dialdehyde occurs. Two equivalents of periodic acid are required. Four electron oxidation.
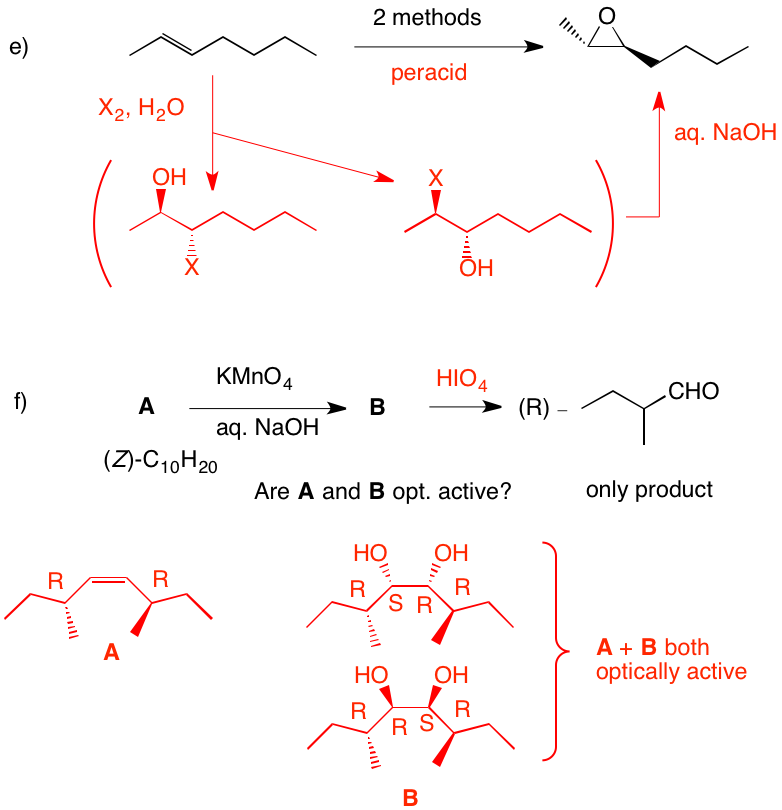
e) There is no indication that the product is optically active; it is racemic. Peracid gives the epoxide. Alternatively, halohydrin formation (X = Cl, Br, I) gives two racemates. The mixture, when treated with base, gives the epoxide.
f) Since the aldehyde is opt. act. and a single product, reconstituting the alkene means that both sides of the double bond are substituted identically. Permanganate adds cis to the (Z)-double bond. Syn addition of two hydroxyl groups to either face of the alkene gives a single diol. Both A and B are optically active and identical. Periodic acid will effect cleavage of the diol B. Why is (E)-A excluded? |