The Sharpless Asymmetric Dihydroxylation
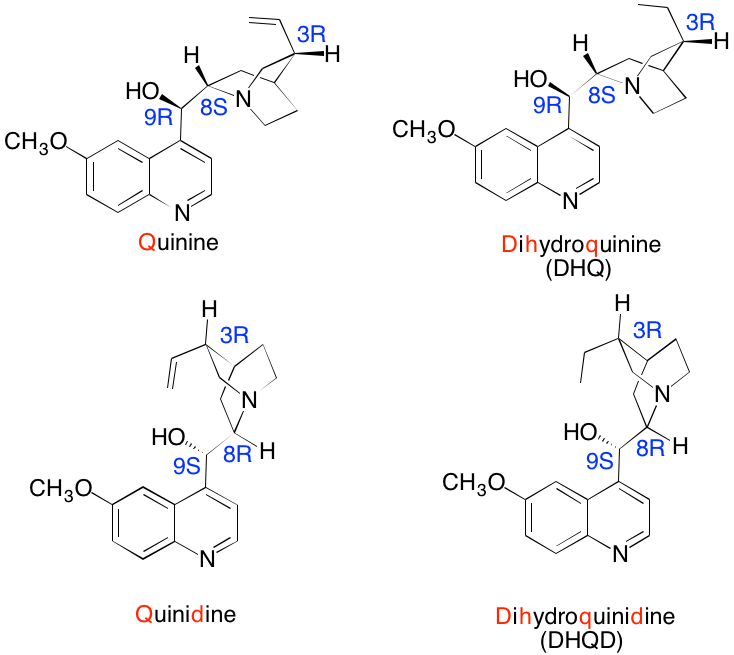
| Quinine (Q) and quinidine (QD), along with their respective dihydro analogs, are isolated from the bark of the Cinchona tree indigenous to Peru. Quinine has been the principle anti-malarial drug for centuries. Additionally, Q is an antipyretic, anti-inflammatory and analgesic agent. On the other hand, QD is used as an anti-arrhythmic drug. Q and QD are optically-active diastereomers of one another. Reduction of the vinyl group of Q produces DHQ; similarly, QD provides DHQD. In the quinine series (Q and DHQ) the aromatic moiety is distal to the vinyl (ethyl) group, each of which is attached to the [2.2.2]-azabicyclooctane ring. On the other hand, in the QD series (QD and DHQD) these groups are proximate to one another. Note that Q and QD (and there respective dihydro derivatives) differ in configuration at both C8 and C9. Reduction of the vinyl group in Q and QD does not affect the R,S-assignment. The structures one the right are the standard method for presenting these alkaloids. Reduction of the vinyl group in Q and QD does not affect the R,S-assignment. The structures one the right are the standard method for presenting these alkaloids. |
|
| The asymmetric catalyst in AD-mix-α (AD = Asymmetric Dihydroxylation) is (DHQ)2PHAL, which is prepared from 2 equivalents of the alkoxide of DHQ and 1 equivalent of the dichloride of PHAL. The linker PHAL is designed so that it can be prepared by nucleophilic, aromatic substitution. The DHQ moieties are drawn in a seemingly unusual perspective. This is done to facilitate drawing an uncluttered structure. The asymmetric ligand has a two-fold axis of symmetry about the green, vertical arrow. As shown, the non-aromatic nitrogen (sp3 hybridized) on the left side of the chiral ligand is in the front of the structure while the one on the right side is in the rear. Rotation about the green arrow by 180o gives the same picture. |
|
| AD-mix-β is (DHQD)2PHAL. Its mode of preparation is the same as described above. Notice that the non-aromatic, sp3 hybridized nitrogen on the left side of the catalyst is now in the rear while the one one the right side is in the front. AD-mix-β which also has a two-fold axis of chirality about the vertical green arrow, has the opposite sense of chirality of AD-mix-α. | 2PHAL-beta.png) |
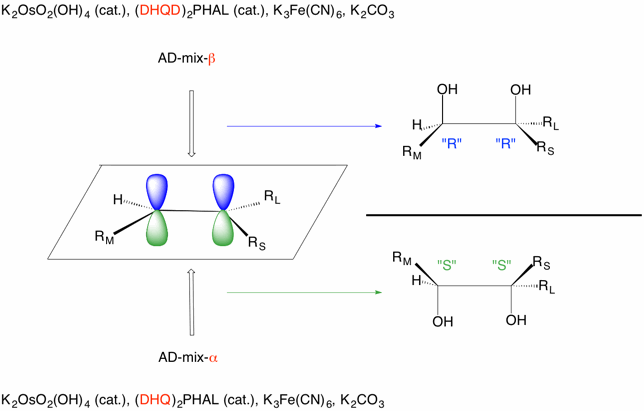
For asymmetric dihydroxylation to be possible, the faces of the the alkene must be enantiotopic. The generic alkene on the right has enantiotopic faces because dihydroxylation leads to two enantiomeric 1,2-diols. (The CIP designations are in " " assuming R = alkyl and RL > RM >RS > H.) AD-mix-α reacts faster with the bottom face of the alkene (green lobes) while AD-mix-β reacts faster with top face of the double bond (blue lobes). (Think green grass below blue skies. Also, alpha goes with "bottom" and beta goes with "top". Pair the words with greater number of letters.) Another mnemonic to remember is that steroids are drawn as shown for estradiol. The beta (top) face is the top. That is the C8-H, methyl and hydroxyl are all of the beta (top) configuration relative to the ring system while the C9-H and C14-H are of the alpha(bottom) configuration.
|
|
2PHAL-alpha.png)