Cahn-Ingold-Prelog Rules: The Inositols
(Powered by Jmol 14.31.0)
Index:
 |
|
 |
 |
 |
 |
 |
 |
 |
|---|
The inositols are cyclohexane hexaols (cyclitols). There are nine possible stereoisomers, the first seven of which, as listed above, are optically inactive. Assigning CIP-R/S-r/s configurations to carbon atoms in the inositols is not trivial. Any carbon lying in a plane of symmetry is stereogenic and achirotopic and it is designated as r/s. All carbons not lying in a plane of symmetry, i. e., all others, are stereogenic and chirotopic and are labeled R/S. Both the 1966 and revised 1982 CIP rules give the same results for the R/S designations for chirotopic carbon atoms for the inositols although the methodologies differ. The earlier CIP rules give different assignments (r/s) for achirotopic carbon atoms from those arising from the latter CIP rules. IUPAC numbering from 1973 is used.
Descriptors for Chirotopic Atoms by 1966 CIP Rules:
But where does one begin? First to be considered are assignments to chirotopic atoms (R/S) by 1966 standards. CIP Rules 0-2 do not apply. Every carbon bears a hydrogen, a hydroxyl group and two, seemingly, identical carbon atoms. At least one knows what the low and top priority groups are. But how do you handle the carbons and give them priorities? One begins with Rule 3, Seqcis precedes Seqtrans. In Fig. 1 the target atom is labelled "t". The configuration of Cab is compared with Ca'b'. If one pair is cis and the other trans, then the priorities at "t" are OH>cis>trans>H. If pairs are cis/cis or trans/trans, Plan B is put into effect. Now compare Cac with Ca'c' (c = c'). If there is no success at this stage, evaluate Cbc vs. Cb'c'. If there is no resolution after all of this, then you are dealing with a carbon that is achirotopic. Cis- and scyllo-inositol have no chirotopic carbon atoms, i. e., three planes of symmetry passing through carbon atoms.. Myo-, epi- and neo-inositol have single planes of symmetry passing through two 1,4-carbon atoms and, accordingly, have four chirotopic carbon atoms. While allo-inositol has a plane of symmetry, it does not pass through any carbon atoms. Therefore, it has six chirotopic carbon atoms. Muco-inositol has two planes of symmetry but only one passes through carbon atoms. It also has four chirotopic carbon atoms.
|
|
|---|
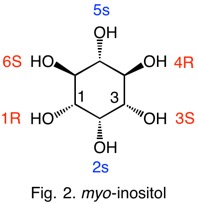
Let's use myo-inositol as an example (Fig. 2). Clearly, C2 and C5 lie in a plane of symmetry. We will deal with the other four chirotopic carbons first. Using C1 as "t", C5,6 is trans and C2,3 is cis. Therefore, C2 has precedence over C3; C1 is of the R-configuration (right-handed rule). Each new chirotopic target atom is treated in the same fashion. Comparison of C1,2 with C4,5 gives C3 an S-configuration. The configurations at C1 and C3 must be of opposite configurations given that they are reflections of the C2-C5 mirror plane. By similar reasoning C4 is R and C2 is S. In the case of myo-inositol, each chirotopic center was determined at the Cab level. This will not always be the case. In neo-inositol C4 and C6 require Cac analysis. While the configuration at C5 in Fig. 2 is the same as the assignment at the top of the page, the assignments for C2 differ. The assignment in Fig. 2 follows the R precedes S ( CIP Rule 5) promulgated in 1966. The discrepancy will be discussed later. In the interim the discussion will focus on mirror planes, axes of rotation and the configuration of chirotopic carbon atoms. |
 |
|---|
Epi- and muco-inositol only differ in configuration at C3 but both of them have a plane of symmetry passing through C3 and C5. For epi-inositol this plane requires that the configuration at C1(R) is the opposite of C5(S) and C2(R) is the mirror image of C4(S). Muco-inositol must obey the mirror image rule but differ from the assignments made for epi-inositol. Thus, C1(R) mirrors C5(S); C2(S) reflects C4(R). Muco-inositol also has a plane of symmetry that passes horizontally through the C1-C2 and C4-C5 bonds. This plane requires C1 to reflect C2 and C4 to reflect C5, which it does. In addition to a vertical plane of symmetry, neo-inositol has a horizontal, two-fold axis of rotation. This axis requires C1 to have the same S-configuration as C2 and C3 to have the same R-configuration as C4 because 180o rotation about this axis creates an identical image. Although allo-inositol has a plane of symmetry, it passes through two bonds and not through carbon atoms. Accordingly, there are six chirotopic carbon atoms. Carbons 2, 1 and 6 have the mirror image configurations of carbons 3, 4 and 5, respectively. D- and L-chiro inositol, the only chiral members of this group, are numbered in the enantiomeric sense requiring Cn in one enantiomer to have the opposite assignment of Cn in the other enantiomer. |
|
|---|
The chart below shows that the chirotopic, stereogenic carbons of the inositols retain the same assignments in the original and revised CIP rules. The differences arise in some of the achirotopic, stereogenic carbons owing to a subtle changes in the rules. These differences are highlighted in red in the yellow panels. As noted earlier myo-inositol by 1966 standards has a 2s,5s configuration. By the 1982 rules it has a 2s,5r configuration. An analysis of neo-inositol provides a good opportunity to compare the methodology of the 1966 rules versus those employed in 1982.
| Inositols/CIP Rules | Original (1966) | Revised (1982) |
|---|---|---|
| cis | ---- | 1s,2s,3s,4s,5s,6s |
| epi | 1R,2R,3s,4S,5S,6s | 1R,2R,3s,4S,5S,6r |
| muco | 1R,2S,3s,4R,5S,6s | 1R,2S,3r,4R,5S,6r |
| allo | 1R,2R,3S,4S,5R,6S | 1R,2R,3S,4S,5R,6S |
| scyllo | ---- | 1r,2r,3r,4r,5r,6r |
| neo | 1S,2r,3R,4R,5r,6S | 1S,2s,3R,4R,5s,6S |
| myo | 1R,2s,3S,4R,5s,6S | 1R,2s,3S,4R,5r,6S |
| D-chiro | 1R,2S,3S,4S,5S,6R | 1R,2S,3S,4S,5S,6R |
| L-chiro | 1S,2R,3R,4R,5R,6S | 1S,2R,3R,4R,5R,6S |
|
|
|---|
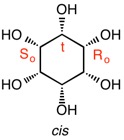 |
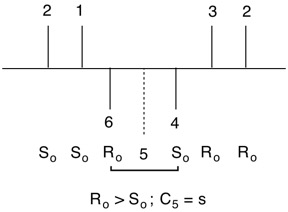
Why does cis-inositol have an all s-configuration? All six centers are equivalent. What is true for one is true for all. For a target carbon (t), the vicinal, clockwise (viewer's right) carbon has descriptor Ro. The counterclockwise, vicinal carbon is So. Since Ro > So, the target carbon has an s-configuration. A similar argument is made for scyllo-inositol. A bold hydroxyl bearing carbon (t) has a hashed bond clockwise to it that bears an Ro-descriptor. The counterclockwise carbon is So. Therefore, the target carbon has an r-descriptor. All carbons bearing hashed bonds are also "r". Try it! In, addition, flipping the structure makes all hashed bonds bold and vice versa. |
 |
|---|
Digraphs of Eight Other Inositols:
Select an inositol. It will appear in the right window. Click on "show descriptors" to display the descriptors for the selected inositol.
Select an Inositol
|
Return to the Top of the Page.
F. E. Ziegler 2017