Chirotopicity and Stereogenicity
[Powered by Jmol 14.31.0]
Ribitol
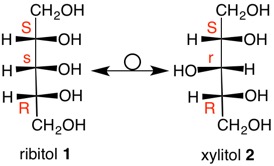 |
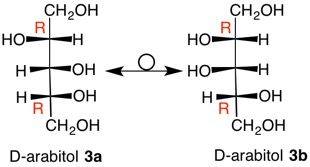
In the majority of asymmetric carbons that one encounters the carbon is both stereogenic and chirotopic, i. e., they define stereochemistry and handedness. But aren't they the same thing? No! Ribitol (1, Fischer projection) is a meso polyol that is optically inactive. A plane of symmetry passes through C3 and its hydrogen and hydroxyl group. The S-configuration at C2 mirrors the R-configuration at C4. Both of these carbons are stereogenic and chirotopic. But what of C3? Inversion of the configuation at C3 does not lead to a mirror image as might be the case with (S)-2-butanol, but rather to a diastereomeric, meso polyol, xylitol (2). Thus, C3 is said to be stereogenic and achirotopic. The lower case "s" at C3 in ribitol refers to its stereochemistry (stereogenicity) and not to chirality. Applying the CIP Rule 5 that states R precedes S, C3 has an s-configuration. Clearly, xylitol has an r-configuration at C3. Now look at D-arabitol 3a, which is chiral and optically active. Both C2 and C4 are of the R-configuration. Inversion of the C3 configuration of 3a gives 3b, which is still D-arabitol. Rotate 3b by 1800 to the other Fischer projection and it will be identical to 3a. Thus, C3 is chirotopic and non-stereogenic. There cannot be a label for C3 because C2 and C4 are identical groups. In years gone by, C3 was known as a pseudo-asymmetric carbon. |
D-Arabitol
 |
|---|
| (4R, 2Z, 5E)-hepta-2,5-dien-4-ol | In the previous discussion the CIP Rule 5 "R precedes S" was discussed. In the accompanying structures, one would be perplexed to provide an R/S assignment to C4 because C1-C3 and C5-C7 decode by CIP rules in exactly the same way. To resolve this dilemma, the CIP Rule 3 "Z precedes E" was introduced. | (4S, 2Z, 5E)-hepta-2,5-dien-4-ol |
|---|