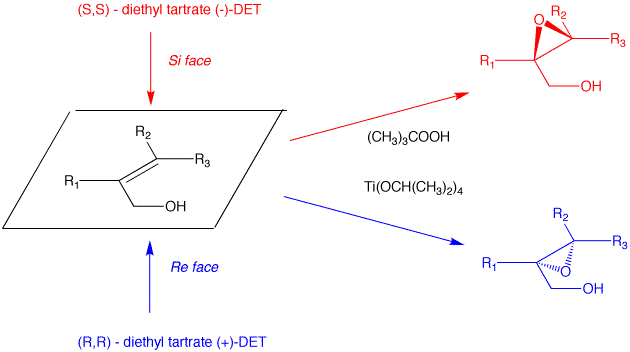
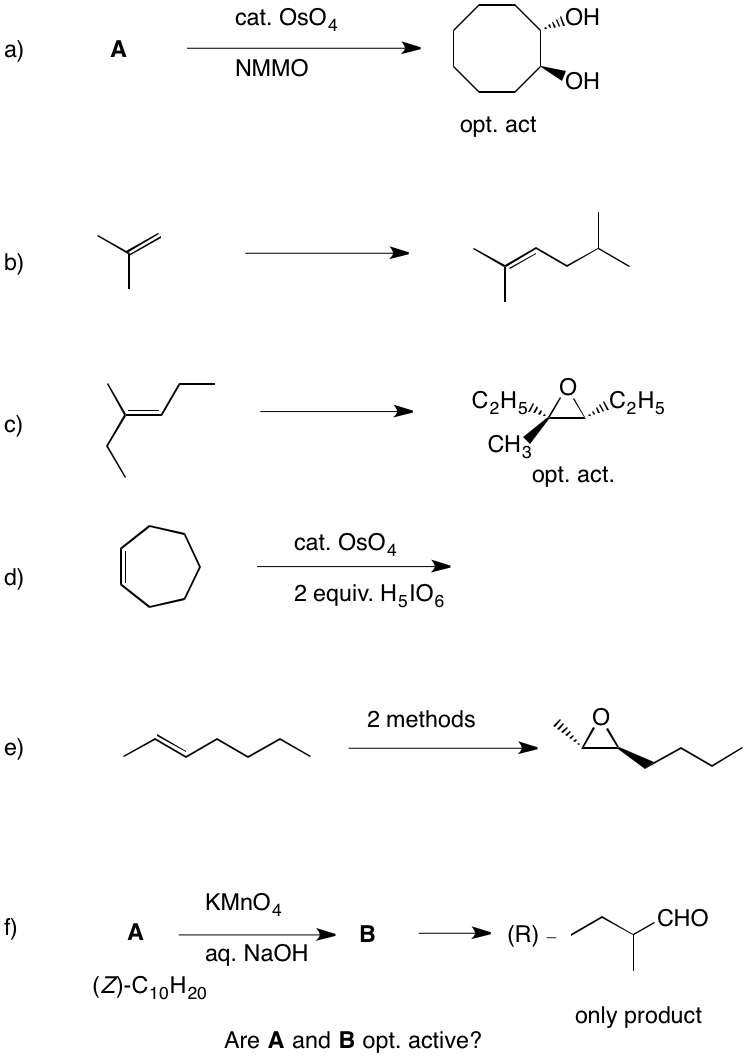
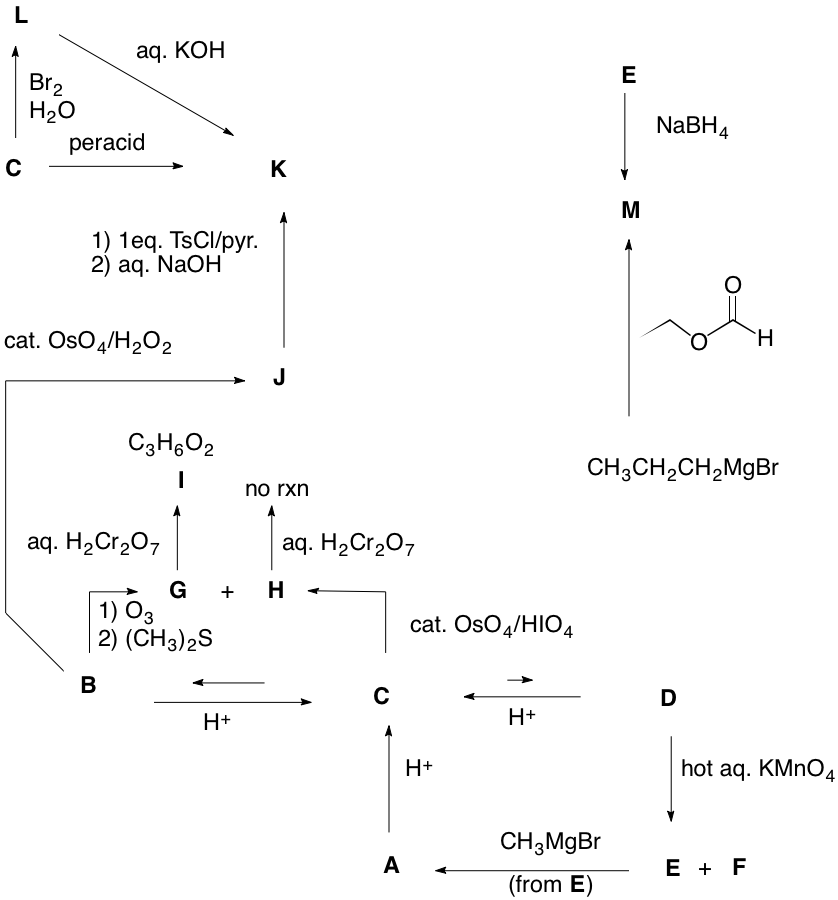
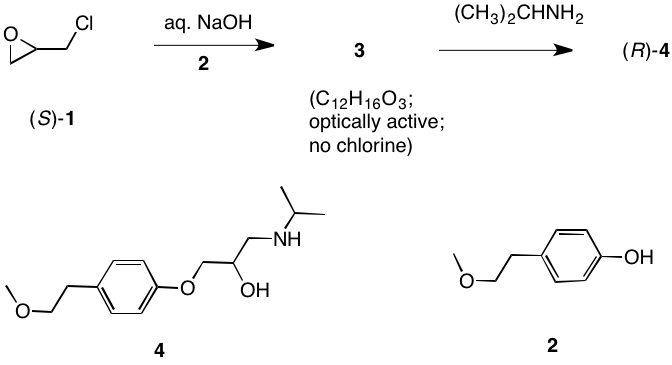
K. Barry Sharpless Co-Nobelist 2001 Asymmetric Epoxidation and Dihydroxylation |
|---|
Reading Assignment:
Sharpless Asymmetric Dihydroxylation
Enrichment Assignment:
Professor Sharpless visits Chem 125
Diethyl ether (ether) may well be
the first organic compound prepared that does not
appear in Nature. For a chemical history of ether click
here. A different
Powerpoint version is here.
Theory
of Etherification, A. W. Williamson, Quarterly
J. Chem. Soc., 1852, 4,
106. [See page 106 in
the .pdf file.]
"The following experiments were made with the view of obtaining new alcohols, by substituting carburetted hydrogen for hydrogen in a known alcohol. Iodide of potassium was readily formed on the application of a gentle heat, and the desired substitution was effected; but, contrary to expectation, the compound thus formed had none of the properties of an alcohol -- it was nothing else than common ether, C4H10O." On Etherification, A. W. Williamson, Quarterly J. Chem. Soc., 1852, 4, 229.