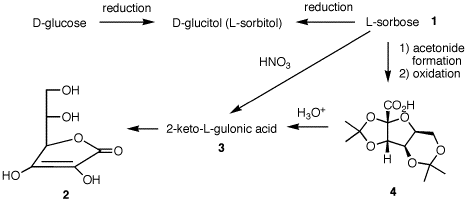
Problem Set 9, Chapter 23
Carbohydrates
Due: Monday, April 18, 2005
Last year's PS9 was so good, here it is again.
The graphic on the left was
conceived by M.
A. Rosanoff in 1906 as a means of
classifying the carbohydrates. It is from this scheme that
the modern pyramidal presentation of the carbohydrates is
derived. Although the left side of the diagram is the
L-series and the right side is the D-series, you will see
D's and L's interspersed. The D's and L's within the circle
were Fischer's
assignments. Check out the links for Fischer-Rosanoff
convention. For more on Emil Fischer, go
here.