
Figure 1
by
M. A. Rosanoff
Emil Fischer's
determination of the relative stereochemistry of
(+)-glucose in 1891 and
his lucky choice of which enantiomer represented (+)-glucose stands
as a landmark achievement in organic chemistry. This accomplishment
put the asymmetric carbon, as championed by van't Hoff and LeBel, on
a firm footing from which there was no retreat.

Figure 1
Fischer also focused on
classifying the aldoses (monosaccharides) into two families: the
d-series and the l-series. The d-series was that group of compounds
that could be related to (+)-glucose [Fig. 1] chemically
while the l-series was related to (-)-glucose. Thus, (+)-gulose is in
the d-series because it and (+)-glucose form the same enantiomeric
aldaric (glycaric or saccharic) acid. In a 1906 paper, M. A. Rosanoff
took issue with this argument of Fischer. Rosanoff formulated
Fischer's position as follows:
"Two aldoses can produce the same dibasic acid only if they belong to the same stereochemical family. That this, however, is erroneous as a general proposition, may be readily seen from the fact that the two enantiomorphous galactoses - plainly belong to the opposite families - yield the same mucic acid." [Fig. 2]
Figure 2
Fischer's scheme must assume that
two enantiomeric aldoses cannot form the same glucose enantiomer.
That is, there is the implicit assumption that the two families or
series are mutually exclusive. Rosanoff proposed a classification
that was ultimately based on the Kiliani homologation and Ruff/Wohl
degradation of aldoses. He named these two series using Greek
descriptors as the δ-family and the λ-family to
distinguish them from Fischer's d- and l-families. In the ensuing
discussion, Fischer's d and l appear in upper case as D and L.
Although the Rosanoff scheme would survive, it did so by replacing
the Greek descriptors with D and L as we understand them today.
In the circular chart by Rosanoff shown below, the vertical axis
separates the λ-series (left side) from the δ-series
(right side). The λ-series contains four D-aldoses
[Fischer designation] while their four mirror image L-aldoses
are contained in the δ-series. In the center of the diagram is
the two carbon, achiral compound, hydroxyacetaldehyde
(HOCH2CHO). Every successive circle homologates the
central structure by -CH(OH)-, that is, a Kiliani synthesis. The
number of structures in each shell doubles by 2n-1 where
the innermost circle is n=1. The outermost circle ( n=5) bears the 16
aldohexoses. The aldoses in each successive circle, proceeding
outward, are the C2-epimers derived from the aldose in the
inner circle with which they are contiguous.
Rosanoff's λ-family Modern L-series Rosanoff's δ-family Modern D-series
If the vertical north-south axis is treated as a
mirror, every structure on the left is the mirror image of the one on
the right. All of the structures on the left have the highest
numbered asymmetric center (most remote from the aldehyde group) on
the left of the Fischer projection. The converse is true on the
right. It is apparent from the Rosanoff scheme that (+)-glucose (10)
is in the δ-family while (+)-gulose (12) is in the λ-family.
As noted earlier, the Fischer designations of D and L were dropped
and Rosanoff's δ became the new D while λ became L. The
left side of the diagram is referred to as the L-series; the right
side as the D-series.
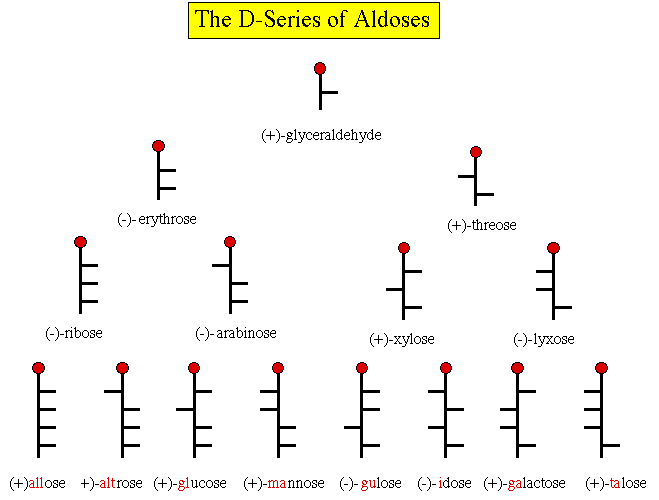
The right side of Rosanoff's circular diagram is usually presented
today as a pyramid shown below. A mirror image pyramid can be
constructed for the left side of the diagram as well. Starting with
glyceraldehyde, Kiliani synthesis gives the aldotetroses, erythrose
and threose. Similarly, the tetroses afford the aldopentoses, which
lead to the aldohexoses. The nonsense line, "All altruists gladly
make gum in gallon tanks" , is a mnemonic to help remember the names
of the aldohexoses. [Fieser & Fieser, Advanced Organic
Chemistry, 1965, pg. 947]. How about a devise for the 7 aldoses
at the top of the chart? Here is a start;
GET
Rest
At
Xanadu
Lodge.
OK, how about GET
Rems
at
X-ray
Lab.
You're on your own!
copyrighted F.E. Ziegler 2002