Problem Set 1
Chapters 1 and 2, Structure, Bonding, Alkanes
Due: Monday, January 28, 2013
|
John Dalton (1766-1844) |
John Dalton's formulation of an Atomic
Theory in the first decade of the
19th century provided a theoretical basis for understanding
chemical behavior. In addition to defining the Law of
Multiple Proportions, he also formulated the Rule of
Greatest Simplicity, which held that water was a binary
compound, OH. (Note: Dalton did not use our modern symbols,
which came to us from Berzelius,
but rather circles that were distinguishable
from one another.) Dalton established the combining masses
of H to O in water as ~1:6. This ratio was later refined to
1:8. The Rule
of Greatest Simplicity, which was
at odds with Gay-Lussac's Law of Combining Volumes of Gases, did not lead to a correct
formulation for the atomic composition of water. Moreover,
although there was agreement regarding the combining masses
of atoms in the first half of the nineteenth century, there
was disagreement as to the unit mass
of the common atoms encountered in organic chemistry:
hydrogen (1), carbon
(2x6 or 1x12), oxygen (2x8 or
1x16). Since hydrogen was the lightest of the elements, it
was assigned a mass of one, a notion that is unrelated to
today's mass of hydrogen owing to the presence of a single
proton in the hydrogen nucleus. Berzelius's proposal of a
mass scale based upon O = 100 would have worked as well. For a Brief History of Organic Chemistry (PowerPoint), click here.
|
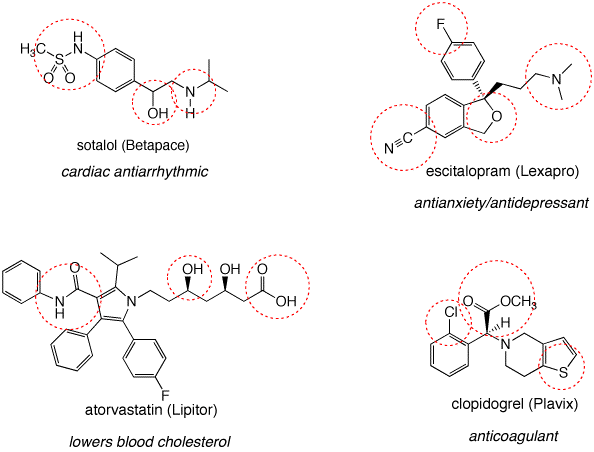
1. Identify the functional groups in the red circles. The front inside cover of your text will be of use. Complete this problem on a copy of this page and attach it to your homework.