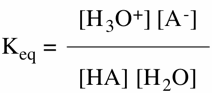pKa - A Discussion
In general chemistry you learned about the pH scale, i.e., pH = -log [H+]. Water has a molar hydrogen ion concentration of [10-7]. Thus, the pH of water is seven. More acidic aqueous solutions, acetic acid or dilute aqueous HCl, have a pH less than seven while aqueous solutions of sodium hydroxide or ammonia have a pH greater than seven. By placing a pure acid HA in water an equilibrium is established wherein the action of the Lewis base water forms a new acid, hydronium ion (Fig. 1). The anion A- is the conjugate base of acid HA and H2O is the conjugate base of hydronium ion, H3O+. In the case of weak acids this equilibrium lies heavily to the left (Keq << 1) and the concentration of HA is usually much less than that of water. Thus, aqueous 0.1M HA is 0.1 M in HA but 55.5 M in water (1000 g/(18 g/M) = 55.5 M) (Fig. 2). Note that Keq is dimensionless. Because the molar concentration of water is much greater than HA, the concentration of water is effectively constant. Therefore, the term Keq[H2O] is constant and defined as Ka with the dimension moles/liter ([M]; Fig. 3). In the same fashion as pH was defined, pKa = -log Ka. This means that if a Brønsted acid (donates protons) has a large pKa it is a very weak acid. If the pKa is small, even negative, it is a strong acid.
Fig. 1 |
Fig. 2 |
Fig. 3 |
While the range of acids dealt with in aqueous medium ranges roughly from -2 to 16 pKa units, in organic chemistry the range is more like -10 to 50. These outlying acids with pKa = < -2, would readily be deprotonated by water while the conjugate bases of "acids" with pKa > 16 would readily deprotonate the "acid" water. Accordingly, many of the pKa's of acids in the table on the right are determined in other solvent than water or by other methods. The absolute values of the pKa's in the range 20 to 50 are not what is important but rather their relative positions. The pKa of ammonia and amines is often reported as 37; a value 35 will due just fine. The conjugate base of an acid with a larger pKa will deprotonate an acid with smaller pKa. Conversely, an acid with a smaller pKa will protonate the conjugate base of an acid having a larger pKa. Consider the amide anion (NH2-). It is formed by the action of sodium metal in liquid ammonia (not aqueous ammonia!). This base will deprotonate any acid with a smaller pKa. Acetylene and terminal alkynes have a pKa of 25. This means that acetylene is a weaker acid than ammonia by ten orders of magnitude. To put it another way, amide anion is ten orders of magnitude more basic than the anion of acetylene. Clearly, as shown below, the equilibrium --- if indeed it can
be called an equilibrium --- lies heavily to the right. The reaction is virtually irreversible. (The sodium counterion has been omitted.) If one were asked what happens when acetylide anion is mixed with liquid ammonia. The answer is nothing other than having a solution of acetylide ion in liquid ammonia. Another way to view this process is to consider two bases, acetylide and amide anion, competing for a proton. Which one wins the day? Amide anion does because it is the stronger base, the conjugate base of a weaker acid. A larger version of the pKa table is here. |
 |