Problem Set 1
Chapters 1 and 2, Structure, Bonding, Alkanes
Due: Monday, January 28, 2013
Solution Set
|
John Dalton (1766-1844) |
John Dalton's formulation of an Atomic
Theory in the first decade of the
19th century provided a theoretical basis for understanding
chemical behavior. In addition to defining the Law of
Multiple Proportions, he also formulated the Rule of
Greatest Simplicity, which held that water was a binary
compound, OH. (Note: Dalton did not use our modern symbols,
which came to us from Berzelius,
but rather circles that were distinguishable
from one another.) Dalton established the combining masses
of H to O in water as ~1:6. This ratio was later refined to
1:8. The Rule
of Greatest Simplicity, which was
at odds with Gay-Lussac's Law of Combining Volumes of Gases, did not lead to a correct
formulation for the atomic composition of water. Moreover,
although there was agreement regarding the combining masses
of atoms in the first half of the nineteenth century, there
was disagreement as to the unit mass
of the common atoms encountered in organic chemistry:
hydrogen (1), carbon
(2x6 or 1x12), oxygen (2x8 or
1x16). Since hydrogen was the lightest of the elements, it
was assigned a mass of one, a notion that is unrelated to
today's mass of hydrogen owing to the presence of a single
proton in the hydrogen nucleus. Berzelius's proposal of a
mass scale based upon O = 100 would have worked as well. For a Brief History of Organic Chemistry (PowerPoint), click here.
|
1. Identify the functional groups in the red circles. The front inside cover of your text will be of use. Complete this problem on a copy of this page and attach it to your homework.

2. Draw resonance structures (if they exist) for the following species. Include all formal charges.
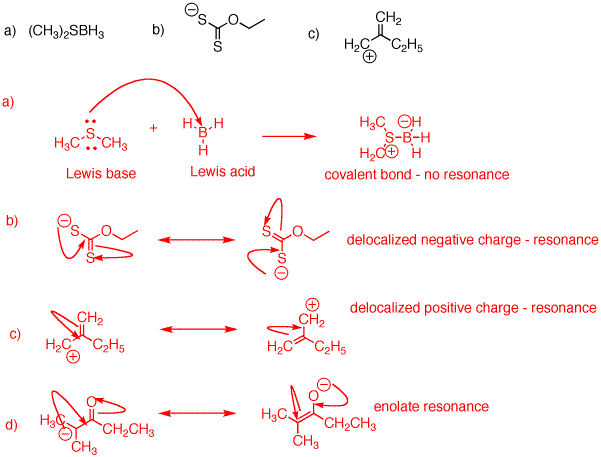
In b) the negative charge is 50% on each of the sulfur atoms. In c) the positive charge is borne equally by the two carbon atoms. In d) more of the negative charge is borne by oxygen (more electronegative) than by carbon.
3. Identify the hybridization (sp, sp2, sp3) of the carbons, oxygen and nitrogen in escitalopram in problem #1 above. Re draw the structure and indicate each site with an arrow.
| All of the benzenoid carbons of escitalopram are sp2 hybridized (planar). The nitrile has both an sp hybridized carbon and nitrogen. All of the remaining carbons are sp3 hybridized, as well as the remaining nitrogen and oxygen, and the remaining nitrogen are sp3 hybridized. |  |
|---|
4. For each of the following acid/base reactions, provide appropriate equilibrium arrows reflecting the position of the equilibrium. For the right side of the equilibrium, provide the conjugate acids and bases. Estimate the equilibrium constant for each reaction. For help on this topic, click here.

5. Arrange the acids in problem #4 in order of increasing acidity (decreasing pKa). The higher the pKa, the less acidic. Thus, the order is methane (50), ethylene (44), ammonia (35), 1-butyne (25), ethanol (15.9), water (15.7) and ethyl mercaptan (ethanethiol, 10.5).
6. Draw an orbital picture of vinylacetylene, (CH2=CHCCH). Identify σ- and π-bonds and hybridization.
| The orbital picture of vinylacetylene is on the right. The two red p-orbitals form the π-bond of the vinyl group (ethylene portion of the molecule). All gray orbitals form σ-bonds. All atoms attached to the two carbons (sp2hybridized) of the red π-bond lie in a plane. The orthogonal sets of blue p-orbitals form two orthogonal π-bonds. The carbon and hydrogen atoms attached to the two carbons of the acetylene portion (blue) of the molecule lie in a linear arrangement. These two carbons are sp-hybridized. |  |
|---|
7. A normal alkane, CnH2n+2, is found to have a
vapor density of 2.16 mg/mL at 100oC and 700 mm pressure.
Using the ideal gas law, determine the structure of the alkane. (In
the early 19th century, the vapor
density of an unknown liquid was compared
to the vapor density of air to determine the liquids molecular
weight.)
| Recall from general chemistry the ideal gas law: PV = nRT, where n = g/M; P (atmospheres), V (liters), n = (moles), g = (grams), M = MW = (grams/mole), T (temperature in oK) and R = (gas constant, 0.0821 l-atm/moles-oK). Since density (d) = mass/volume; d = g/V. Since PV = nRT or PV = gRT/M, then d = g/V =PM/RT. Transposing, M = dRT/P. At a given temperature and pressure, d is proportional to M by a factor of R. P = 700 mm Hg/760 mm Hg = 0.921 atm.; T = 100oC + 273o = 373oK. M = dRT/P = (2.16) x (0.0821) x (373)/0.921 = 71.8. M.W. = 72. Based upon the formula, the alkane is a completely saturated, acyclic alkane. There are n carbons at mass 12 and 2n +2 hydrogens at mass 1 in the compound. Therefore, (12)n + [(1)(2n +2)] = 72 or 14n = 70; n = 5. The alkane is C5H12. Since it is a normal alkane, it is unbranched. Ans: n-pentane. |
How to manipulate JSmol structures n-pentane |
|---|