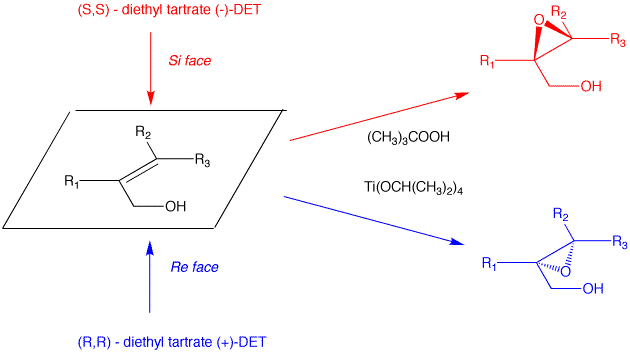
K. Barry Sharpless Co-Nobelist 2001 Asymmetric Epoxidation and Dihydroxylation |
|---|
Reading Assignment:
Enrichment Assignment:
Diethyl ether (ether) may well be
the first organic compound prepared that does not
appear in Nature. For a chemical history of ether click
here. A different
Powerpoint version is here.
Theory
of Etherification, A. W. Williamson, Quarterly
J. Chem. Soc., 1852, 4,
106. [See page 106 in
the .pdf file.]
"The following experiments were made with the view of obtaining new alcohols, by substituting carburetted hydrogen for hydrogen in a known alcohol. Iodide of potassium was readily formed on the application of a gentle heat, and the desired substitution was effected; but, contrary to expectation, the compound thus formed had none of the properties of an alcohol -- it was nothing else than common ether, C4H10O." On Etherification, A. W. Williamson, Quarterly J. Chem. Soc., 1852, 4, 229.
1. Each of the following reactions is missing a reactant, reagent and/or product. Provide the missing items along with an explanation.
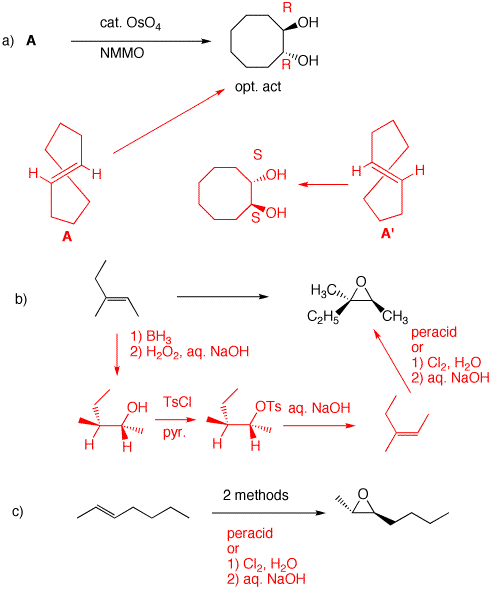
a) The diol is optically active (R, R) and trans. Since OsO4 adds syn to a double bond, A cannot be cis-cyclooctene, which would provide the cis-diol. A is the enantiomer of trans-cyclooctene shown. Its enantiomer, A', would afford the S,S-enantiomer. The reaction of OsO4 with the double bond of trans-cyclooctene can only react with the external face.
b) Direct epoxidation would give the diastereomeric epoxide. The alkene geometry must be inverted. Hydroboration adds water syn and anti-Markovnikov. Removing the elements of water anti E2 as TsOH gives the (Z)-alkene. Epoxidize by either of two procedures to give the racemic epoxide.
c) See the last step of part b.
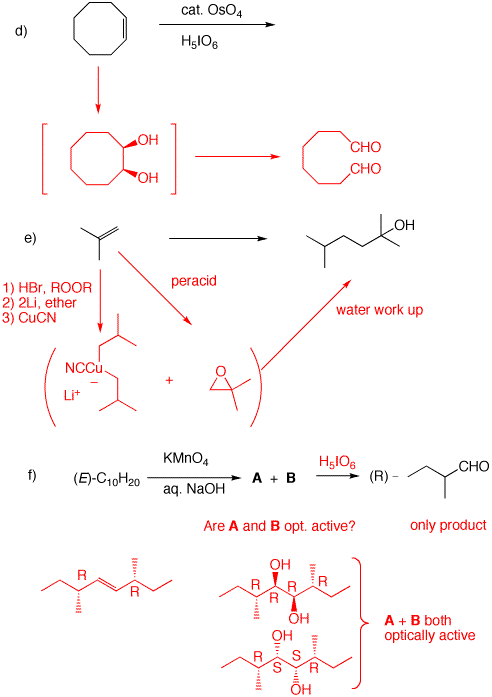
d) cis-1,2-Cyclooctanediol is formed in situ. Cleavage to the dialdehyde occurs. Two equivalents of periodic acid are required. Four electron oxidation.
e) Isobutylene must be used twice and a carbon-carbon bond must be formed. The epoxide is the electrophile and the cuprate reagent is the nucleophile. Cuprates may be generated from Grignard reagents (or organolithium reagents). The problem with magnesium cations, and Grignard reactions with highly substituted epoxides, is that they can effect rearrangement of epoxides. In this instance isobutyraldehyde can form and act as an electrophile toward the magnesium reagents. Attack occurs in an SN2 fashion at the less substituted carbon of the epoxide. f) Since the aldehyde is opt. act. and a single product, reconstituting the alkene means that both sides of the double bond are substituted equally. Permanganate adds cis. Both diols are optically active diastereomers. |
|
|---|
2. A graduate student requires two related compounds: (±)- 2,2-dimethyl-3-isobutyloxirane (A) and (±)-2,5-dimethylhexan-2,3-diol (B). She has available to her isobutane as the only source of carbon. She decides to synthesize 2,5-dimethyl-2-hexene C, an alkene that can serve to provide both A and B.
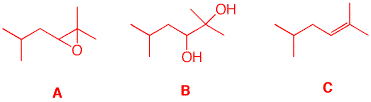
a) Show her (your) synthesis of C. Free radical bromination is highly selective for tertiary hydrogens over primary ones (~1600:1, tertiary over primary. With 9 primary hydrogens and only one tertiary hydrogen, the selectivity is ~160:1). Elimination gives isobutylene. Carbon-carbon bond formation is provided in 1e. E1 elimination of the tertiary alcohol affords the more substituted alkene C. There are two methods available for forming A and B from C. The conversion of A to B and B to A is shown below.
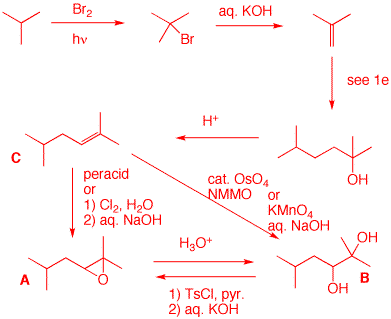
b) Show how she can convert A into B and B into A.
See part a.
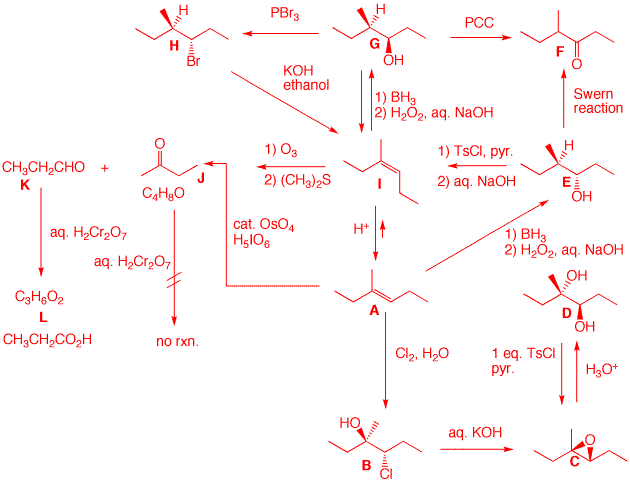
3. Identify each of the structures below. Pay attention to stereochemistry. Pay attention to the equilibrium arrows in the equilibrium between A and I. Ignore any constitutional isomers that may arise. All compounds are C7 as seen in the data for J and K. They are formed from A and I by reactions that cleave double bonds. A and I are alkenes. A is more stable than I based on the position of the equilibrium catalyzed by acid. [Ignore the possibility of formation of the alternative trisubstituted alkenes from the intermediate tertiary cation.] J must be a ketone; inert to chromic acid. Only 2-butanone is possible for J. K is propionaldehyde because it is oxided to a carboxylic acid L, propionic acid. Piecing J and K together A and I become (E)-3-methyl-3-hexene and (Z)-3-methyl-3-hexene, respectively. The rest of the structures fall into place knowing the reactions and stereochemistry.
4. Two oxidative pathways are shown below for the conversion of (E)-3-hexene 1 to propionaldehyde 4. The total electron change must be the same in both routes. Illustrate the electron change for each of the four steps.
| a) Polarize the double bond in alkene 1 as + and -. [Review here.] Carbons 2 and 3 in 2 are δ+ relative to oxygen. Net 2-electron oxidation. Diol 2 to two aldehydes is a two electron oxidation. If the C2-C3 bond of diol 2 is broken [mentally] heterolytically, species 5 and 6 are formed. Species 5 is a protonated aldehyde. Species 6 requires the loss of the two electrons to become 5. Break two bonds of ozonide 3 heterolytically to form 7 and 8. Species 7 is an aldehyde while two electrons must be added to the positive oxygen of 8 (and two protons) to give the hydrated form of an aldehyde. Protonation and hydration are electroneutral. Since both routes are 4-electron oxidations, alkene 1 forming ozonide 4 is a 6-electron oxidation. | 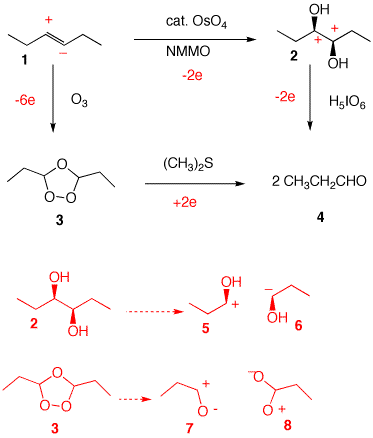 |
|---|
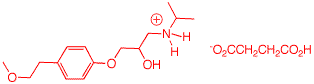
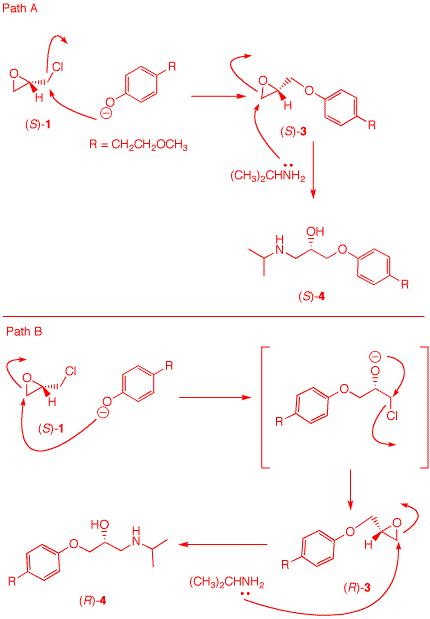
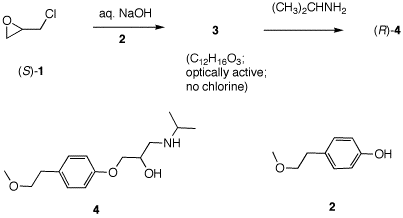
5. Toprol (4; Lopressor; Metoprolol) is a β-blocker for the treatment of hypertension. It is a racemic drug sold as its monosuccinate salt. A seasoned chemist wants to prepare Toprol as its (R)-enantiomer using the synthesis of the racemate. She follows the steps shown on the right. a) Why is Toprol a racemate? When prepared from racemic 1, 4 is a racemate because the carbon bearing the OH group has both enatiomers present. b) Draw a structure of the
(±)-succinate salt. c) What is the role of aq. NaOH? Check
here. The strong base
NaOH forms the anion of the phenol 2.
|
f) How can she prepare (S)-4 without resorting to the use of (R)-1? Use the amine as the first nucleophile and then employ the phenoxide. |
|---|