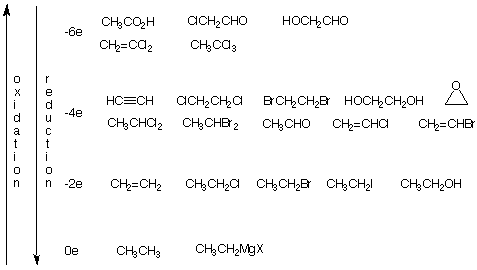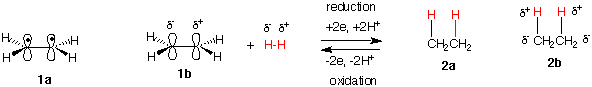
An appreciation of the oxidation level of a carbon skeleton can aid you in determining whether a chemical transformation requires oxidation, reduction or a non-oxidative/reductive (electroneutral) reaction. It is not necessary to assign formal charges to all atoms but rather to compare the reactant with the product. Although all the examples are C2 compounds, this method can be applied to any carbon compound.
Reduction/Oxidation: The Basics
Free Radical Bromination of Ethane
Formation and Reactions of Organometallics
Alcohols, Aldehydes and Carboxylic Acids
Reduction/Oxidation: The Basics
The atoms and electrons in chemical reactions are conserved. That is to say, all reactions must be balanced in atoms and electrons. In the study of organic chemistry, there are many occasions when reactions are not balanced because some atoms are not considered an integral part of the process or our focus is on the carbon compound undergoing a chemical reaction. In a properly electronically-balanced chemical reaction, the electrons may be gained (reduction) by a substrate from a reducing agent. At the same time, the substrate is acting as an oxidizing agent as the reducing agent loses electrons (oxidation). A useful technique is to be able to recognize whether or not an organic molecule is being oxidized or reduced, or no oxidation or reduction has occurred.
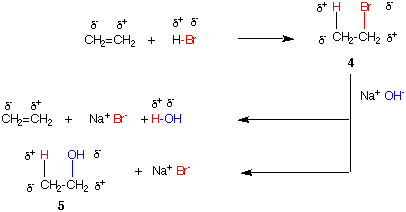
Structure 1a is a typical representation of ethylene. Structure 1b is a polarized form of ethylene where the pair of electrons are localized in one of the p-orbitals of the double bond. We can consider one of the carbons as being negative and the other as being positive. Similarly, H2 can be considered having one hydrogen δ- (partially negative) and the other δ+. If a molecule of H2, which may also be considered two protons and a pair of electrons (all of which are in red), is added to the double bond of ethylene, ethane (2a) is formed. Because carbon is more electronegative than hydrogen, the new C-H bonds in ethane are polarized as shown in ethane 2b. One of the carbon atoms of ethylene has remained negative while the other one has gained two electrons (reduction). Hydrogen, the reducing agent, has become oxidized because both of the hydrogens from H2 are now δ+, or positive if you wish. From the substrate perspective, ethylene has been reduced to ethane (2 electron reduction). For every reduction, there must be an oxidation. In this case, H2 is being oxidized (loss of 2 electrons) to 2H+. Ethylene is said to be 2 electrons higher in oxidation level (state) than ethane. If the process were conducted in the opposite direction, ethane would be oxidized to ethylene (2 electron oxidation).
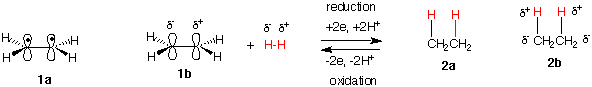
The trio of hydrocarbons ethane (2), ethylene (1) and acetylene (3) are related as shown below. Any process that interconverts ethane and acetylene must involve a change of four electrons.

Free Radical Bromination of Ethane
The free radical bromination of ethane 2 affords ethyl bromide (bromoethane) 4 and HBr. [For a detailed account of free radical chain reactions, see the Alkane module in ORGO.] In this reaction a hydrogen in ethane is replaced by bromine. Because the relative electronegativities are in the order H < C < X (halogen), the carbon undergoing reaction is oxidized (δ- --> δ+). What is being reduced? Bromine, which in effect is Br- and Br+, become negative in both ethyl bromide and HBr. Ethyl bromide is said to be 2 electrons higher in oxidation level (state) than ethane. Because both ethylene (see above) and ethyl bromide are 2 electron oxidation products of ethane, the two of them must be at the same oxidation level. How can this point be verified? [That the conversion of 2 to 4 occurs via a radical mechanism is irrelevant to the analysis being applied. The comparison is made between 2 and 4 and it is unrelated to the mechanism of the reaction.]
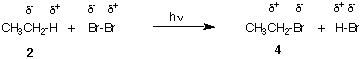
Addition of HBr to Ethylene, E2 Elimination, SN2 Substitution
The addition of HBr to ethylene occurs by initial addition of a proton to ethylene to form an ethyl cation followed by capture of bromide ion to form ethyl bromide. There is no change in oxidation level of the carbon framework. The polarities of the new bonds in ethyl bromide are the same as the polarities of the atoms in the reactants.
Two reactions of NaOH with ethyl bromide can be imagined: E2 elimination or SN2 substitution. In the E2 process the elements of hydrogen bromide are eliminated. The electronegative groups (Br-, OH-) remain negative, electropositive groups (H+, Na+) remain positive. The addition of HBr and its elimination are the reverse of one another. The second reaction, the SN2 process, has electronegative Br- exchanged for electronegative OH- to form ethanol (5). There is no net effect on the polarity of the carbon to which these two groups were attached. We may conclude that ethylene, ethyl bromide and ethanol are at the same oxidation level. In fact, any species H+ Y- that adds to ethylene will be at the same oxidation state as these three compounds as long as Y is more electronegative than carbon. The hydration of ethylene to form ethanol is a case in point.
Formation and Reactions of Organometallics
The reaction of magnesium metal with ethyl bromide 4 in the presence of ether forms the Grignard reagent, ethyl magnesium bromide (6). In ethyl bromide the carbon of the C-Br bond is δ+. By inserting Mgo in the C-Br bond, the carbon becomes δ- (or -1) owing to the electropositive nature of Mg+2.
The carbon in question is reduced by 2 electrons while Mgo is oxidized to Mg+2. When water is added to the Grignard reagent, acid/base chemistry ensues. Because CH3CH2- is a stronger base than Br-, the proton of water reacts to form ethane (see pKa's). The overall conversion of ethyl bromide 4 to ethane 2 is a reduction of the carbon skeleton.
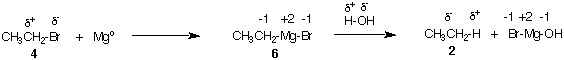
The halogenation of ethylene is a 2 electron oxidation of the carbon skeleton. Consider the bromination shown below. The dibromide 7 has both bromine atoms more electronegative than carbon whereas Br2 had one of the bromine atoms positive. Ethylene is oxidized, bromine is reduced. If dibromide 7 is reduced with zinc metal (see the section above), the zinc is oxidized to Zn+2 and ethylene is formed. On the other hand, if an elimination occurs with base, the oxidation level of vinyl bromide 8 remains the same as dibromide 7 (see Addition of HBr to Ethylene, E2 Elimination, SN2 Substitution for review). How can you
tell?
Compare vinyl bromide 8 with ethylene. If H2 were added to the double bond of 8 (a 2 electrom reduction), ethyl bromide would be formed which, as stated earlier, is at the same oxidation level as ethylene. Alternatively, to form vinyl bromide 8 from ethylene, an electropositive hydrogen would have to be replaced by electronegative bromine, a process that is a net 2 electron oxidation of carbon. Remember, absolute charges are not being assigned to atoms in as much two compounds are being compared to determine if the carbon skeleton is more, or less, oxidized.
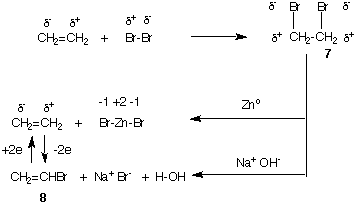
Alcohols, Aldehydes and Carboxylic Acids
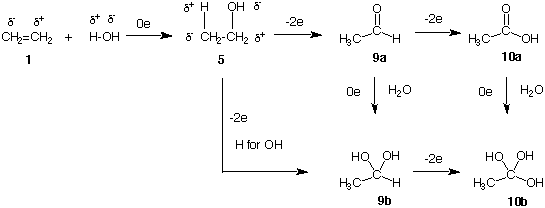
The formation of ethanol 5 by the addition of water to ethylene 1 is a non-oxidative process (Addition of HBr to Ethylene, E2 Elimination, SN2 Substitution). Acetaldehyde (9a) is formed by a 2 electron oxidation of ethanol and, in turn, acetic acid (10a) is the 2 electron oxidation product of acetaldehyde. A carbonyl group (C=O) is polarized with positive charge on the carbon and negative charge on oxygen. Addition of H2O (H+ and OH-), a non-oxidative process, across the carbonyl group of acetaldehyde 9a gives the hydrated form of acetaldehyde 9b. Acetaldehyde 9a and hydrate 9b are at the same oxidation level. Now imagine exchanging a C1 hydrogen of ethanol (5) for an OH group to form 9b. This process has increased the oxidation level (more positive) of C1 of ethanol by loss of 2 electrons. The two cycles, 5 --> 9a --> 9b and 5 --> 9b, must sum to the same number of electrons. Similarly, the conversion of 9b to the hypothetical triol 10b, which can be viewed as the hydrated form of a carboxylic acid, is a 2 electron oxidation.
The chart below includes the examples that have been discussed in addition to others. Convince yourself that each compound within the designated oxidation level is interconvertible without oxidation or reduction and that interconversions between levels involve oxidation or reduction. You may wish to complete the chart up to -14 electrons where acetic acid is oxidized to two molecules of CO2. In fact, all of these carbon compounds can be converted to CO2.