Due: Monday, November 1,
2010
|
Ozone
In 1840, Christian
Friedrich Schönbein
(1799-1868) discovered ozone (Gr.; odorant), the sharp
odor produced by electrical discharges. Seven years later
(1847) he observed that ozone oxidizes organic compounds
but not to their ultimate products of oxidation, carbon
dioxide and water. [Two years prior, he had spilled
nitric and sulfuric acid on his Frau's apron in her
kitchen. The apron, made of cotton, combusted and thus
was discovered gun cotton, nitrocellulose. Schönbein
also observed that hydrogen peroxide (Threnard; 1818) is
oxidized to oxygen gas in the presence of hemoglobin.
] In the period 1903-1916, Carl
Dietrich Harries (1866-1923),
an assistant to both Hofmann (of the eponymous
elimination and rearrangement) and Fischer (of projection
and carbohydrate fame) at Berlin, published some 80
papers on the reactions of ozone with organic compounds.
His interest was stimulated by the reaction of ozone with
rubber, a process that causes rubber to become hard and
brittle. These studies led to the analytical and
synthetic uses of ozone. From 1904-1916 he was a
professor at Kiel. Disenchanted with academic life, he
became Director of Research for Siemens and Halske, the
German company co-founded by the electrical pioneer,
Werner von Siemens, his father-in-law. Not surprisingly,
Siemens went into the business of producing ozone
generators. The studies of Rudolf
Criegee (1902-1975;
Karlsruhe)
produced a unified mechanism for the process
of ozonolysis.
M.
Rubin, Bull. Hist. Chem., 2001, 26,
40.
M. Rubin, Helv. Chem. Acta,
2003, 86, 930.
|
|
Reading assignments:
a)The alkene module
in
ORGO.
b)
Ozonolysis
module.
How do I approach solving problems
like #2---5? Here
is a step-by-step analysis of a typical problem.
|

Vladimir Vasilovich
Markovnikov
(1838-1904)
|
|
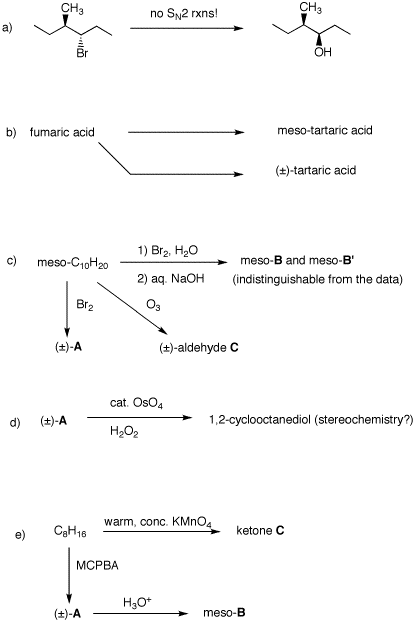
1. Provide the missing information in
each of the following problems: reagents or unknown
structures. Explain your reasoning.
|
|
2. Optically active monoterpene
A reacts with 2 molar equivalents of hydrogen to
produce diastereomeric, disubstituted cyclohexanes
B and C, both of which are optically
inactive. Compound B has a smaller heat of
combustion than C. Ozonolysis and dimethyl sulfide
reduction of A affords the compound on the right
as a reaction product as its (R)-enantiomer
[Hint: Count carbons]. What are the
structures A-C? Explain and illustrate.
|
|
|
3. Compound A reacts with
Br2 in CCl4 to give B. The
intermediate in this reaction (C) is a meso
species. Ozonolysis of A affords only
2-methylpropanal (isobutyraldehyde). What are the
structures A-C? Explain and illustrate. Pay
attention to stereochemistry.
|
|
4. Compound A
(C10H20) undergoes ozonolysis to
produce a
single,
optically active compound
(S)-B. The reaction of compound A
with Br2 in CCl4 provides a
single,
optically active compound
C. What are the structures of A-C? Show
their stereochemistry. Show your reasoning.
|
|
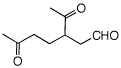
5. Compound A,
C7H12, [Degree
of Unsaturation?] affords
a
single
ketoaldehyde B upon ozonolysis and dimethyl
sulfide reduction. Hydrogenation of A gives
methylcyclohexane. Treatment of A with HBr in the
presence of
peroxide
gives two stereoisomeric bromides, C and D.
Compound C reacts with
C2H5ONa/C2H5OH
to give E while under the same conditions,
compound D gives mainly A and some of
compound E. Ozonolysis of E gives a single
dialdehyde F. What are the structures of
A-F? Explain and illustrate. Pay attention to
stereochemistry.
|


