Due: Monday, November 2,
2009
|
Ozone
In 1840, Christian
Friedrich Schönbein
(1799-1868) discovered ozone (Gr.; odorant), the sharp
odor produced by electrical discharges. Seven years later
(1847) he observed that ozone oxidizes organic compounds
but not to their ultimate products of oxidation, carbon
dioxide and water. [Two years prior, he had spilled
nitric and sulfuric acid on his Frau's apron in her
kitchen. The apron, made of cotton, combusted and thus
was discovered gun cotton, nitrocellulose. Schönbein
also observed that hydrogen peroxide (Threnard; 1818) is
oxidized to oxygen gas in the presense of hemoglobin.
] In the period 1903-1916, Carl
Dietrich Harries (1866-1923),
an assistant to both Hofmann (of the eponymous
elimination and rearrangement) and Fischer (of projection
and carbohydrate fame) at Berlin, published some 80
papers on the reactions of ozone with organic compounds.
His interest was stimulated by the reaction of ozone with
rubber, a process that causes rubber to become hard and
brittle. These studies led to the analytical and
synthetic uses of ozone. From 1904-1916 he was a
professor at Kiel. Disenchanted with academic life, he
became Director of Research for Siemens and Halske, the
German company co-founded by the electrical pioneer,
Werner von Siemens, his father-in-law. Not surprisingly,
Siemens went into the business of producing ozone
generators. The studies of Rudolf
Criegee (1902-1975;
Karlsruhe)
produced a unified mechanism for the process
of ozonolysis.
M.
Rubin, Bull. Hist. Chem., 2001, 26,
40.
M. Rubin, Helv. Chem. Acta,
2003, 86, 930.
|
|
Reading assignments:
a)The alkene module
in
ORGO.
b)
Ozonolysis
module.
How do I approach solving problems
like #1---5? Here
is a step-by-step analysis of a typical problem.
1. An optically active compound A
(C8H14) reacts with catalytic
OsO4 and stoichiometric
H2O2 to form (R,R)-diol
B. Ozonolysis and dimethyl sulfide reduction of
A forms OHC(CH2)6CHO. What
are the structures of A and B?
Explain.
|

Vladimir Vasilovich
Markovnikov
(1838-1904)
|
|
The formula of
A indicates 2 degrees of
unsaturation (DU's). That A reacts
with OsO4 and O3 indicates at least
one double bond. The dialdehyde product of ozonolysis
indicates the alkene was 1,2-disubstituted and contained
in a ring. The ring and double bond account for the 2
DU's. Deconstructing the dialdehyde to the alkene whence
it came, leads to cis- or trans-cyclooctene for
A. That A forms
a chiral (R,R)-diol B means that
A is also chiral.
A must be an enantiomer of
trans-cyclooctene, which is resolvable. Cis-cyclooctene,
which is achiral, would lead to an achiral 1,2-cis-diol.
See diagram on the right. The two enantiomers of
A,
A1 and
A2, cannot interconvert
because the double bond cannot pass through the ring.
This also means that only one face of the double bond is
exposed to syn-hydroxylation. Syn addition to a
trans-double bond gives hydroxyl groups that are trans on
the ring. Clearly, B2 is
B and A2
is A.
|
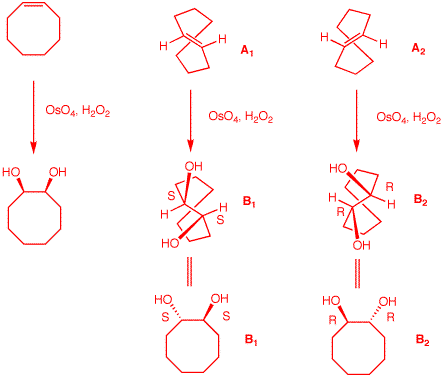
|
|
2. Compound A,
C7H12, [Degree
of Unsaturation?] affords
a
single
ketoaldehyde B upon ozonolysis and dimethyl
sulfide reduction. Hydrogenation of A gives
methylcyclohexane. Treatment of A with HBr in the
presence of
peroxide
gives two stereoisomeric bromides, C and D.
Compound C reacts with
C2H5ONa/C2H5OH
to give E while under the same conditions,
compound D gives mainly A and some of
compound E. Ozonolysis of E gives a single
dialdehyde F. What are the structures of
A-F? Explain and illustrate. Pay attention to
stereochemistry.
|
DU = 2.
The reaction with O3 suggests an
alkene and hydrogenation gives
methylcyclohexane. The double bond must be
trisubstituted because there is a
single keto aldehyde
formed. A must be
1-methyl-1-cyclohexene. Peroxide and HBr
produces bromine radicals that add to the less
substituted end of the double bond of
A. C
and D are cis and trans
2-methyl-1-bromocyclohexane. But which one is
which? The base treatment to give E2
elimination gives the answer.
For E2 elimination to occur, the
cyclohexane ring must be in a conformation
having the bromine axial. The less stable
conformation of trans isomer
C has the bromine and one β-hydrogen
axial. This loss of HBr affords alkene
E. On the other hand, the
more stable conformation of the cis-isomer
D has an axial bromine and
two axial hydrogens. With small bases such as
ethoxide, the Saytzeff rule applies, more of the
more substituted alkene A
and less of
E.
|
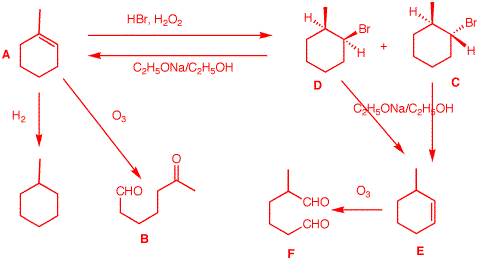
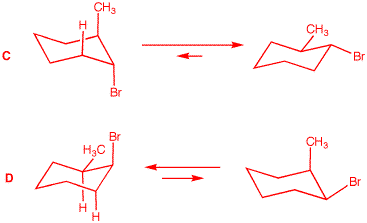
|
|
|
3. Compound A reacts with
Br2 in CCl4 to give B. The
intermediate in this reaction (C) is a racemic
species. Ozonolysis of A affords only propanal
(propionaldehyde). What are the structures A-C?
Explain and illustrate. Pay attention to
stereochemistry.
Since
ozonolysis of A gives only
propionaldehyde (CH3CH2CHO),
A is either (E)- or (Z)-3-hexene. A
bromonium ion is the intermediate in the bromination of
alkenes. The bromonium ion from (E)-3-hexene forms a
racemic mixture (d,l-pair) of intermediates. The
intermediate from (Z)-3-hexene is achiral and meso. Anti
addition of bromine to (E)-3-hexene leads to
meso-3,4-dibromohexene. Anti addition of bromine to
(Z)-3-hexene leads to
d,l-3,4-dibromohexene.
|
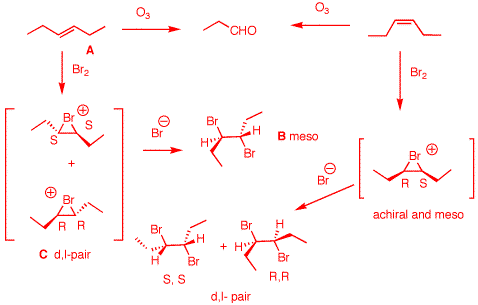
|
|
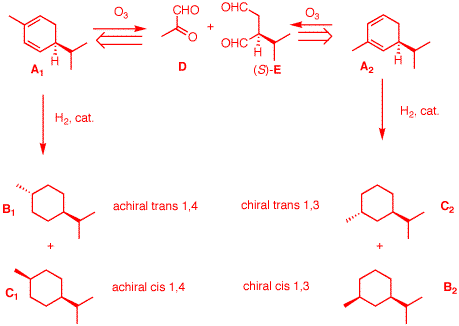
4. Optically active terpene A
reacts with 2 molar equivalents of hydrogen to produce
diastereomers B and C, both of which are
optically inactive. Compound B has a smaller heat
of combustion than C. Ozonolysis and dimethyl
sulfide reduction of A affords pyruvaldehyde
D (C3H4O2,
Google
it) and (S)-isopropylsuccindialdehyde E
(OHCCH(i-Pr)CH2CHO; tartaric acid =
2,3-dihydroxysuccinic acid). What are the structures
A-E? What are the sign and value of the optical
rotation of A (Review PS4)?
Explain. A
terpene has ten carbons. This info distinguishes the
structure from some multiple of C10. There are
only two ways to piece D and
E back together --
A1 or
A2. Two equivalents of
hydrogen convert the diene to a pair of cyclohexanes.
Only B1 and
C1 are optically inactive
(plane of symmetry); B2 and C2 are
optically active. [Note:
B1 and
B2 have the lower heat of
combustion over their respective diastereomers because
they have a more negative heat of formation (more
stable)]. Summary: A =
A1;
B =
B1;
C =
C1.
From PS4, #6, A is (S)-α-Phellandrene ([α]D
= +86o). Based upon PS4,
#7, it
could be (S)-α-Phellandrene ([α]D
= +217o)
|
|
|
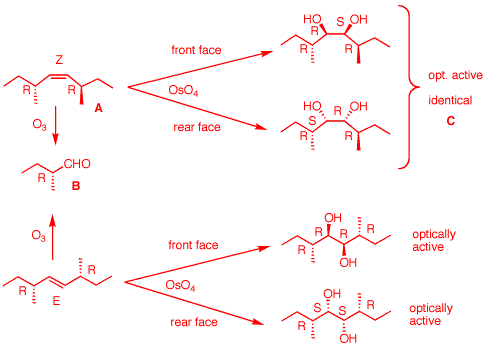
5. Compound A
(C10H20) undergoes ozonolysis to
produce a
single,
optically active compound
(R)-B. The reaction of compound A
with ethereal OsO4 or aqueous KMnO4
provides a
single,
optically active compound
C. What are the structures of A-C? Show
their stereochemistry. Show your reasoning.
Compound
A has 1 DU. A
is an alkene because of its reactions. Since only a
single, optically active compound
(R)-B is formed upon ozonolysis,
B must be C5 and have a
center of asymmetry. There is only one solution:
(R)-CH3CH2CH(CH3)CHO.
All that remains is to determine the geometry of the
double bond. We have an arrangement of a
1,2-disubstituted double bond flanked by two asymmetric
centers of the (R)-configuration. When dihydroxylation
occurs, there will be two chiral hydroxyl centers flanked
by the two (R) centers, or R-X-X-R, where X is a hydroxyl
center. Imagine that the double bond is (Z). Then syn
addition of the two hydroxyls will give R-R-S-R and
R-S-R-R, which are identical and optically active.
Imagine that the double bond is (E). Then syn addition of
the two hydroxyls will give two
compounds: R-R-R-R and R-S-S-R. Both compounds are
optically active. Therefore, the double bond is (Z). To
assist you, think about syn addition to (E)- and
(Z)-2-butene.
|
|
|
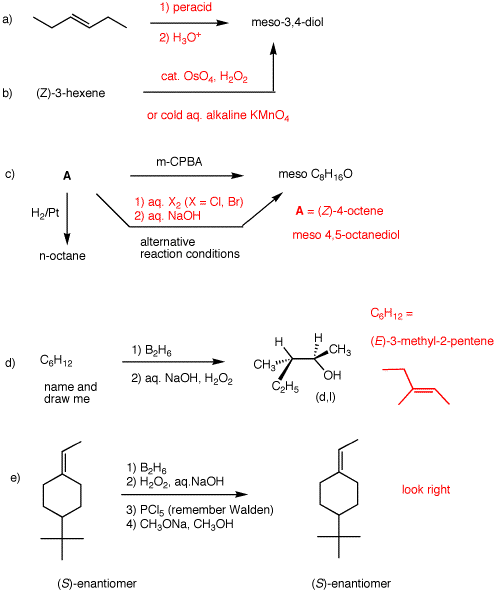
6. Provide the products, reagents,
and/or reagents as required in each of the following
problems. Explanations are required for all.
|
a) We have no
direct way to add two hydroxyl groups anti to a double
bond. Syn epoxidation followed by acid catalyzed,
SN2 opening of the epoxide is a solution.
b) Straight forward syn-epoxidation.
c) A is a normal chain
(hydrogenation data). A is
C8H16 because epoxidation with
peracid (no inversions) adds one oxygen atom syn. Since
C8H16O is meso,
A must be (Z)-4-octene. The
alternative reaction sequence has two inversion
(SN2) reactions.
d) C6H12 is an alkene from the
reaction conditions. The hydroboration sequence adds
water to an alkene in an anti-Markovnikov sense. The
alkene must be trisubstituted. It appears from the
structure of the alcohol that the elements of water have
been added trans, which means the alkene is not
(Z)-3-methyl-2-pentene but rather
(E)-3-methyl-2-pentene.
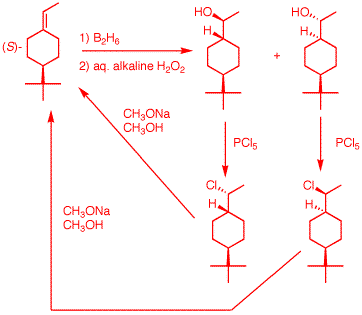
e) This alkene can be optically active. See PS4, #5b. The
tert-butyl group is the highest priority; the hydrogen
the lowest. The methyl on the double bond has priority
over the hydrogen on the double bond. This may seem like
a futile synthesis because you are converting the
starting material into itself. Well, it is. But it is a
good stereochemical excercise. Hydroboration adds water
anti-Markovnikov in a syn manner. There are two possible
stereoisomers; water added either cis or trans to the
tert-butyl group. The hydroxyl groups can be converted to
chlorides with SN2 inversion (See PS5 Solution
Set for a mechanism). Now E2 elimination of
HCl in an anti manner returns the starting enantiomer.
Had a tosylate of the alcohol been used as a leaving
group, the (R)-enantiomer would have formed upon
elimination because tosylate formation does not involve a
change in stereochemistry.
|








