 |
|
(This photograph is in the hallway across from 110 SCL)
|
Reading and Enrichment Assignments:
Problem Set 2 - Solution Set
Chapter 3, Alkanes
Due: Monday, September 21, 2009
(This photograph is in
the hallway across from 110 SCL) In 1885, as an addendum to a paper on
acetylenic compounds, Baeyer proposed that cyclopentane
was the least
strained of the cycloalkanes.
While he accepted the idea that the carbon atoms in
cycloalkanes were tetrahedral, he treated the
cycloalkanes as though they were flat. He argued that
there is only one cyclohexane carboxylic acid, not two
(axial and equatorial) as was predicted by a chair
cyclohexane.

Reading and Enrichment
Assignments:
a. Work through How to Draw Cyclohexanes (PowerPoint)
b. The Conformation Module in the Study Aids will give you a good overview of the subject of conformation.
c. View The Evolution of Formulas and Structure in Organic Chemistry During the 19th Century (PowerPoint).
1. Redraw (line angle formula) and name (IUPAC)
the hydrocarbon in this problem. For a dynamic view click
here.
For a static view click here.
How
to manipulate Jmol structures. [What
if there are two different longest chains? Check
here.]
IUPAC Rule 2.6 for
hydrocarbons states that if there is more than one longest chain, the
one with the greater number of substituents prevails. The hydrocarbon
is clearly a nonane (longest chain). Is it
3,6-diethyl-2,5,5-trimethyl nonane or is it
6-ethyl-3-isopropyl-5,5-dimethyl nonane? Clearly the first nonane has
five substituents while the second one has only four. The first one
prevails.
2. Compound A (MW=139.19), a 1,4-disubstituted cyclohexane, has the following composition: C, 69.03%; H, 9.41%; N, 10.07%. The difference in conformational energy for the two chair conformations of A is 0.4 kcal/mol. Using the A-value data (Energy Differences Between ..... Cyclohexanes), determine the structure of A. Illustrate and explain. What is the conformational energy difference for the stereoisomer of A, ---namely A'. Explain and illustrate. Show the chair comformations of A and A' with the appropriate equilibrium arrows to illustrate the major and minor conformations. Label each conformation with its energy. The first order of business is to determine the molecular formula of the compound. Does compound A contain only C, H and N? No! The sum of the percentages adds up to 88.51%. Since oxygen is determined by difference it must constitute 11.49% of the remaining matter. For the calculation:
The formula is C8H13NO. M.W. calculated:
139.19, which agrees with the given value. [Note: A compound
composed of these four elements must, if it has an odd number of
hydrogens, have an odd number of nitrogens. Check
here.] Compound
A is a 1,4-disubstituted cyclohexane.
Cyclohexane is C6H12. Subtracting two hydrogens
for the positions of the two substituents leaves a cyclohexane
nucleus of C6H10. Subtracting:
C8H13NO - C6H10 =
C2H3NO for the sum of the composition of the
two substituents. Linking to the A-values tables shows only one
functional group containing a single nitrogen, namely, cyano (-CN):
0.2 kcal/mol. We are left with CH3O- for the composition
of the remaining substituent, which is the group methoxy
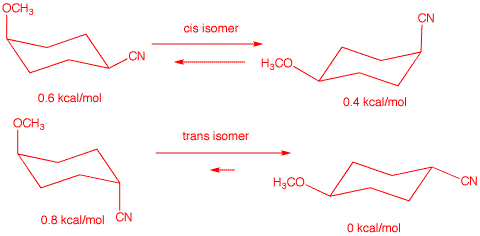
(CH3O-): 0.6 kcal.mol. The energy difference agrees with
the given value. Compound A is the cis-isomer
because the trans isomer A' would have an energy
difference between its two chair conformations of 0.6 kcal/mol + 0. 2
kcal/mol -0 kcal/mol = 0.8 kcal/mol. See below:
3. Predict the heat of formation of n-nonane using the data presented
here.
Explain. The heats of
formation of the n-alkanes differ by approximately -5 kcal/mol per
-CH2- group increase. Since n-octane has ΔHfo
= -49.8 kcal/mol, n-nonane would be expected to have a value of ΔHfo
= ~ -55
kcal/mol.
4. Using the heats
of formation tables,
explain the difference in the heats of formation of cis- and
trans-1,3-dimethylcyclohexane. The difference in the heat of
formation of these stereoisomers is the same as the difference in
their heats of combustion. Justify and illustrate with a
diagram.
Not surprisingly, the
cis-1,3-isomer is more stable (ΔHfo
= -44.1 kcal/mol) than the 1,3-trans-isomer (ΔHfo
= -42.2 kcal/mol). The trans isomer has one axial methyl
group while the 1,3-cis-isomer has none. There difference in
ΔHfo is 1.9 kcal/mol, close to the value
of 1.8 kcal/mol used for equatorial and axial methyl
cyclohexane (Look
here).
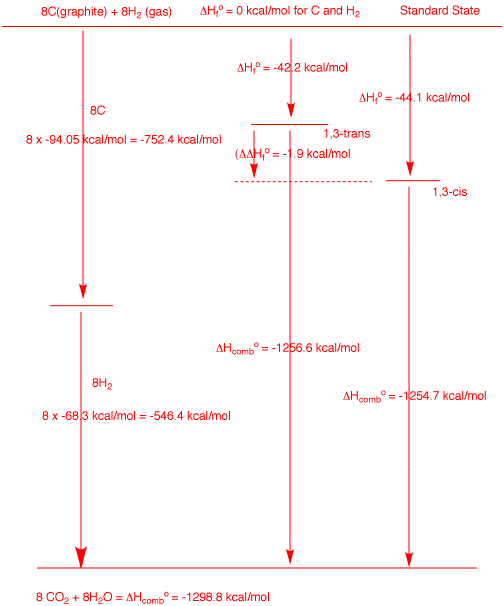
These two isomers have the molecular formula
C8H16. The maximum amount of heat (ΔHo)
that can be generated from combusting 8 moles of graphite
and 8 moles of hydrogen is -1298.8 kcal/mole (see the left
side of the diagram). The trans and cis isomers liberate
-1256.6 and 1254.7 kcal/mol of heat, respectively, upon
combustion. Their respective heats of formation are simply
the difference of each of these numbers from the maximum
value. Clearly from the diagram the difference in the heats
of formation of each isomer is equal to the difference of
their heats of combustion.
5. Calculate the heat of
combustion of cyclopentane using the data (ΔHfo
of cyclopentane, CO2 and H2O) in the
heats
of formation tables.
Compare your value with
the value in Table 3-5.
From the Tables: ΔHfo
(cyclopentane) = -18.3 kcal/mol; ΔHfo
(CO2) = -94.05 kcal/mol; ΔHfo
(H2O) = -68.3 kcal/mol. Cyclopentane =
C5H10. ΔHfo (max) =
5 x [(-94.0) + (-68.3)] = -811.75 kcal/mol.
ΔHcombo (cyclopentane) = ΔHmaxo
(cyclopentane) - ΔHfo (cyclopentane)
ΔHcombo (cyclopentane) = -811.8 - (-18.3)
= -793.4 kcal/mol
You can use a diagram as in #4. Your text lists a value of 3320
kJ/mol. 3320kJ/mol x 4.18 kJ/Kcal = -794.3 Kcal/mol. Close
enough!
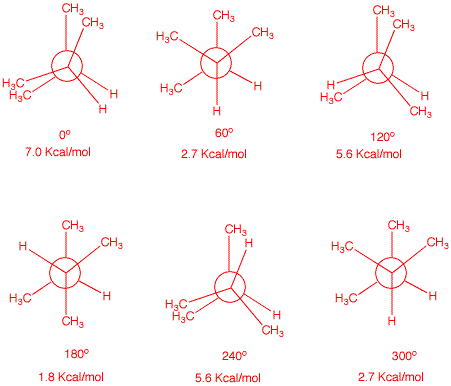
6. Draw Newman projections for the eclipsed and staggered
conformations of 2,3-dimethylbutane viewed along the
C2-C3 axis. Calculate the energy of each
conformation, both staggered and eclipsed.
The Newman projectiions on
the left below have C2 in the front and C3 in
the rear. The degrees refer to clockwise rotation of C3
about the C3 axis. The energies are based upon the
following data: H/H, gauche, 0 kcal/mol; CH3/H, gauche, 0
kcal/mol; CH3/CH3, gauche, 0 kcal/mol; H/H,
eclipsed, 1 kcal/mol; CH3/H, eclipsed, 1.3 kcal/mol;
CH3/CH3, eclipsed, 3.0 kcal/mol. The Jmol
structures on the right match the Newman projections.
Move
the Jmol structures
to see the Newman projections. If you are having trouble with these
energy issues, go
here and do methane
through butane in the Conformation Module.
7. The structures on the right represent
norborane. a) Provide its IUPAC name. b) Identify the bridgehead carbons by
number. (The numbers on the far right are unrelated to the
IUPAC numbering system.) c) Click on the Jmol logo, select
"style", "labels" and then "with atom numbers". Only the
numbered hydrogens appear. d) Measure the C(1)-C(6)-C(12) and the
C(1)-C(2)-C(3) bond angles. (Click on "How to manipulate
Jmol structures" for instructions on measuring.) e) Measure the corresponding H-C(2)-H and
H-C(6)-H bond angles in d). What has happened to these bond
angles as a consequence of the C-C-C bond angles? f) Measure the same angles for C(2) of
the unstrained hydrocarbon, propane. g) Make a chart of the six measurements.
Explain the trend. How
to manipulate Jmol structures. 7a.
Bicyclo[2.2.1]heptane. Its in your text. pg. 118,
6th ed. Compound Propane
C(1)-C(2)-C(3) Propane
H-C(2)-H Norbornane
C(1)-C(2)-C(3) Norbornane
H-C(2)-H Norbornane
C(1)-C(6)-C(12) Norbornane
H-C(6)-H
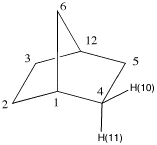
7b. Bridgehead carbons are 1 and 12.
7d. Measurements give 93.5o and 103o,
respectively.
7f. Measurements give 108.2o and
109.7o, respectively.
7g. Chart: Propane is unstrained compared to norbornane. The
C-C-C bond angle is slightly larger than the H-C-H bond
angle in propane. Compare these two values with the bond
angle of 109.5o in methane. The C(1)-C(2)-C(3)
bond angle (103o) in norborane is contained in
both a 6- and 5-membered ring. It is more strained than the
same bond angle in propane. At the same time, its
corresponding H-C(2)-H bond angle increases
(108.2o). The C(1)-C(6)-C(12) is contained in two
5-membered rings. Its bond angle has been reduced to
93.5o while its H-C-H bond angle has increased to
109.7o, greater than the bond angle in methane.
In the chart the C-C-C bond angles are in blue; the H-C-H
bond angles in green. As you move down the Compound column,
the C-C-C bond angles contract while the corresponding H-C-H
bond angles increase.