(How to manipulate JSmol structures)
Propane
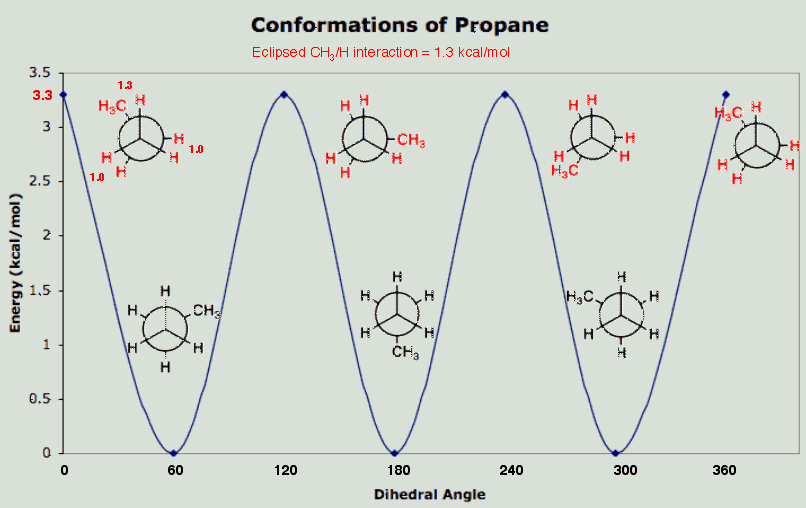
If we take the staggered ethane model and replace a hydrogen atom with a methyl group in such a way that the new methyl group and the attached carbon of "ethane" are staggered, we now have all staggered propane. View the staggered conformation of propane down the C1-C2 axis and the C3-C2 axis. Note that all groups are staggered. If you view down the C1-C2 axis, you see the C3 methyl group bisecting the H-C1-H bond angle. This staggered conformation having the H-C1-C2-C3 dihedral angle (60o) is taken as 0 kcal/mol. If we rotate about the C1-C2 axis by 60o, which is the same as rotating about the C2-C3 axis by 60o, we now have eclipsed propane. Examine the eclipsed propane model. One C-C axis shows an eclipsed conformation; the other C-C axis shows a staggered conformation. This eclipsed conformation (i.e., eclipsed-staggered) lies 3.3 kcal/mol higher in energy than the staggered conformation (i.e., staggered-staggered). The three eclipsing interactions are C-H/C-H, C-H/C-H, and C-H/C-CH3. The value of the C-H/C-H interaction (1 kcal/mol) comes from the eclipsing interaction in ethane. Thus, the C-H/C-CH3 interaction must be 1.3 kcal/mol.
Measure the C-C-C and H-C2-H bond angles in staggered propane. What has happened to them relative to the C-C-H and H-C-H bonds in ethane?
If you are finished, advance to n-butane.