How to manipulate JSmol structures
Butane
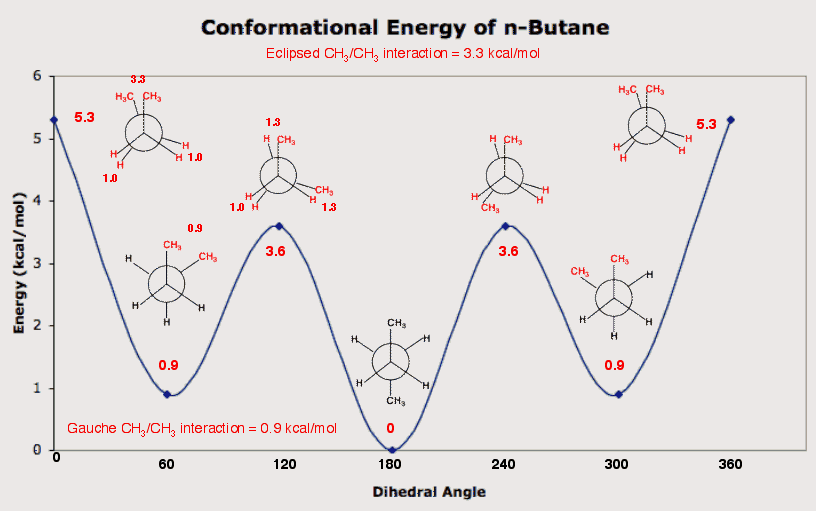
n-Butane is the smallest normal (straight chain) alkane that need not have all its carbon atoms in a plane. Ethane and propane must meet this condition. The best way to assess important steric interactions in n-butane is to examine rotation about the C2-C3 bond of n-butane. Consider the C2-C1 bond and the C3-C4 bond of n-butane as the hour and minute hands of a clock with the C2-C3 bond as the axis about which the hands of the clock pivot. If one hand is considered as fixed on the numeral twelve for the sake of reference, then six o'clock (C1-C2-C3-C4 dihedral angle = 180o ) is represented by the anti conformation (staggered). Here C1 and C4, which may be considered as substituents on the C2-C3 bond, are as far apart from one another as possible. Also see that the three pairs of contiguous carbons, C1-C2, C2-C3 and C3-C4 are staggered. The C1-methyl group bisects a H-C3-H bond angle and the C4-methyl group bisects a H-C2-H bond angle. This arrangement is taken as 0 kcal/mol (cf., propane). One other all-staggered conformation is at two and ten o'clock. At dihedral angles of 60o and 300o (-60o) the gauche (skewed) conformation is formed. This all-staggered (convince yourself) is higher in energy than the all-staggered anti conformation. The C1-methyl group bisects the C4-C3-H bond angle and conversely the C4-methyl group bisects the C1-C2-H bond angle. These arrangements are not separate interactions but a single one. The C1- and C4-methyl groups are close to one another and create torsional strain (C1-C2-C3-C4 dihedral angle = 60o). The gauche conformation is 0.9 kcal/mol higher in energy than the anti conformation.

In addition to the two
staggered conformations, anti and gauche, there are two eclipsed
conformations. One is at noon (dihedral angle = 0o) and
the other is at four or eight o'clock (dihedral angle =
120o and 240o). The eclipsed conformations
are formed by rotating the staggered conformations about the
C2-C3 bond. In so doing, the
C2-C3 substituents become eclipsed while the
C1-C2 and C3-C4
substituents remain staggered. Confirm this statement for
yourself. (In reality, all the C-C bonds and C-H bonds are
rotating at all times. All permutations will not be considered.)
Consider the
120o
eclipsed conformation. It
has two pairs of CH3-C/C-H bonds eclipsed and one
C-H/C-H bond interaction. How many kcal/mol above the anti
conformation does this eclipsed conformation lie?
From ethane,
a C-H/C-H eclipsed interaction was shown to be 1 kcal/mol. From
propane,
a CH3-C/C-H eclipsed interaction was shown to be
~1.3
kcal/mol. Therefore, the
120o eclipsed conformation of butane is 3.6 kcal/mol
higher in energy than the anti conformation. In the
0o
eclipsed conformation there
are two C-H/C-H and one CH3-C/ CH3-C
eclipsed interactions. Given that this conformation is 5.3
kcal/mol higher in energy than the anti conformation, what is the
approximate value of an eclipsed CH3-C/
CH3-C interaction? Each of the C-H/C-H eclipsed
interactions is worth 1.0 kcal/mol. Thus by difference, the
eclipsed CH3-C/ CH3-C interaction
is
worth 3.3 kcal/mol. Return
to the Conformation Module.
.