Research Activities of the Tully Group
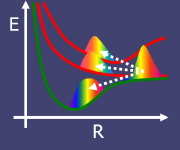 Theories of chemical reaction dynamics are usually based on two
assumptions. The first is the Born-Oppenheimer assumption: electrons
adjust instantaneously to the slower movement of atoms, so that the
atoms can be considered to move along a "potential energy surface"
derived from a single electronic state. The second is the assumption
that the atomic motion can be described by classical mechanics. These
assumptions form the basis of the conventional "molecular dynamics"
computer simulation method. However, there are a great many important
chemical processes for which one or both of these assumptions are
invalid.
Breakdown of the
Born-Oppenheimer assumption is the rule
rather than the exception in electron transfer reactions,
photochemistry, and reactions at metal surfaces. Classical mechanical
treatment of atomic motion is inadequate in situations such as proton
transfer where quantum mechanical zero-point motion or tunneling
effects may dominate.
Theories of chemical reaction dynamics are usually based on two
assumptions. The first is the Born-Oppenheimer assumption: electrons
adjust instantaneously to the slower movement of atoms, so that the
atoms can be considered to move along a "potential energy surface"
derived from a single electronic state. The second is the assumption
that the atomic motion can be described by classical mechanics. These
assumptions form the basis of the conventional "molecular dynamics"
computer simulation method. However, there are a great many important
chemical processes for which one or both of these assumptions are
invalid.
Breakdown of the
Born-Oppenheimer assumption is the rule
rather than the exception in electron transfer reactions,
photochemistry, and reactions at metal surfaces. Classical mechanical
treatment of atomic motion is inadequate in situations such as proton
transfer where quantum mechanical zero-point motion or tunneling
effects may dominate.
While a full quantum mechanical treatment of electronic and atomic motion would be desirable, this is impractical at the present except in the cases of the very simplest chemical systems. In order to realistically describe complex systems, our strategy is to develop methods for introducing the most crucial quantum mechanical effects into an otherwise classical molecular dynamics framework, while self-consistently including feedback between the quantum and classical degrees of freedom. These methods show promise of greatly extending the range of validity of the molecular dynamics approach, while retaining its advantages: practicality, applicability to complex systems, and the ability to "watch" the motions of individual atoms, on the computer screen, as they participate in a chemically reactive event.
These techniques can be applied fruitfully in a number of different fields. The rates and pathways of energy flow in condensed phases and at surfaces, frequently controlling factors in materials processing and catalytic reactions, can be elucidated. Electron transfer reactions in liquids or at the liquid-solid interface are ripe for study, as are electron and proton transfer reactions in biological systems. The competition between thermal and non-thermal reaction pathways in photochemistry can be explored, including transformations induced by high power lasers. Our objective is not merely to reproduce experimental results. We hope to uncover the underlying mechanisms; in other words, why did the experiment turn out the way it did? In addition, we can examine behavior that cannot feasibly be studied experimentally and make quantitative predictions that give guidance to the experimentalist in the design of future experiments.
E-mail: john.tully@yale.edu
Phone: 203-432-3934 (Tully); 203-432-6068 (Group); 203-432-6144 (FAX)
Last Modified: Sept. 2012.
