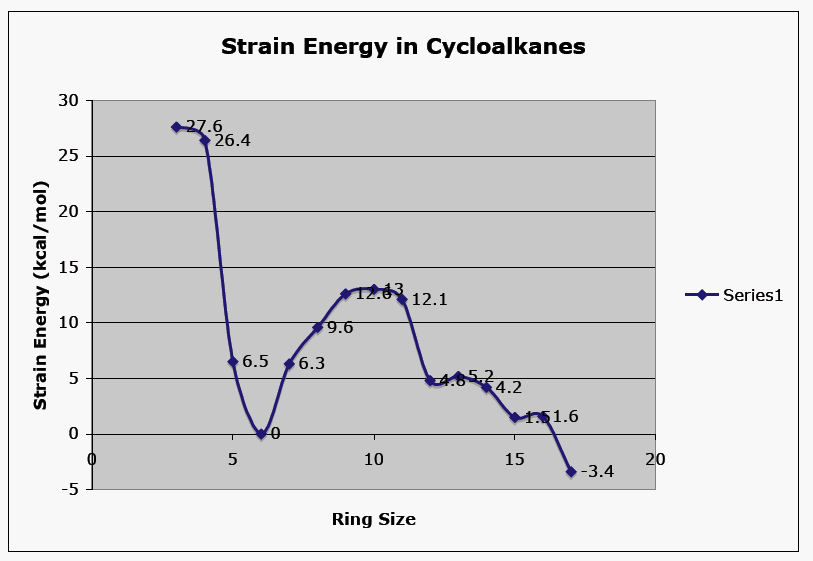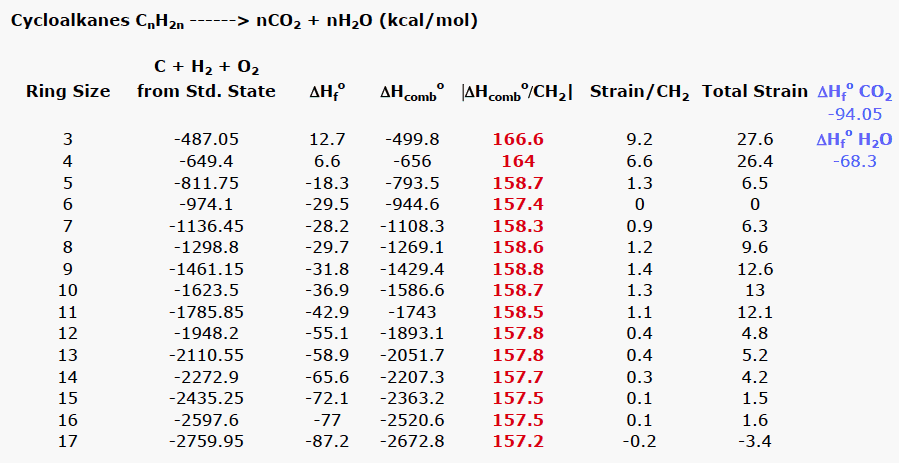
Cycloalkanes, unlike acyclic alkanes, possess properties that create either strain or transannular effects that raise their energy. Small cyclic compounds are confronted with ring strain issues while medium-sized rings suffer from transannular effects. In spite of Baeyer's reasoning for cyclopentane to be the least strained of the cycloalkanes, it is cyclohexane that is the least strained. How does thermochemistry give the correct result? Table 1 lists all the cycloalkanes from cyclopropane (C3) successively through cycloheptadecane (C17). Consider cyclopropane (C3H6) as representative. When 3 moles of carbon (graphite) and 3 moles of hydrogen are combusted, 487.05 kcal/mol of heat are liberated at 25oC and 1 atm. pressure. However, combustion of a mole of cyclopropane liberates 499.8 kcal/mol. The difference between these two numbers is the heat of formation (ΔHfo) of cyclopropane, +12.7 kcal/mol. Both cyclopropane and cyclobutane, both with positive heats of formation, are less stable than the atoms from which they are formed. Dividing |-499.8| kcal/mol by three (the number of methylene groups) gives the absolute strain per methylene group, 166.6 kcal/mol. What does this number mean relative to an unstrained methylene group? What is an unstrained methylene group worth?

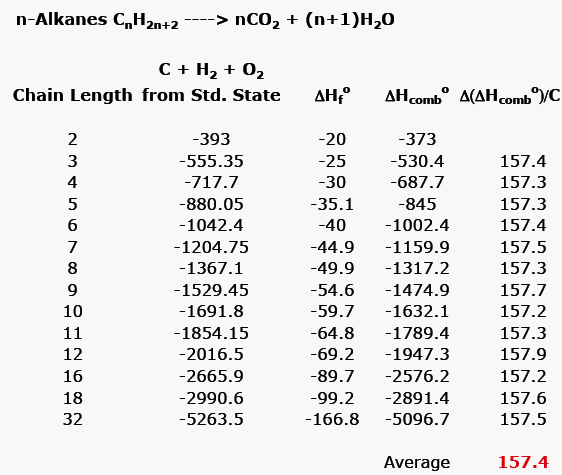
Normal chain alkanes are unstrained because the are not constrained in a ring. Table 2 provides the heat of combustion of a successive, homologous series of n-alkanes, ethane through dodecane, in addition to several higher homologs, C16, C18 and C32. The difference between successive homologs is an absolute value of ~157 kcal/mol. In the case of C16, C18 and C32, the difference is the average when the difference in ring size is greater than one methylene group. The average of all of these values is 157.4 kcal/mol, the heat of combustion of a hypothetical, unstrained methylene group.
Now return to Table 1 and cyclopropane. The difference between the strain in the methylene group of cyclopropane (166.6 kcal/mol) and the unstrained standard (157.4 kcal/mol) affords the absolute value of 9.2 kcal/mol/CH2. Therefore, the strain for cyclopropane itself is found by multiplying 9.2 kcal/mol/CH2 by 3, the number of methylene groups. Each cycloalkane has its strain energy determined in the same fashion. Of all the red values in Table 1, only the value for a methylene group in cyclohexane has the value of an unstrained methylene group, 157.4 kcal/mol. The total strain in cycloalkanes drops rapidly after cyclopropane and cyclobutane to reach a minimum with cyclohexane and rises again with cycloheptane (see graph below). Medium-sized rings, C8-C11, suffer from transannular interactions. There is a drop in strain in larger rings. Looking at the graph, one might think that cycloheptadecane is more stable than cyclohexane because it has a total strain energy of -3.4 kcal/mol. Notice that the gap in the heat of formation between cycloheptadecane and cyclohexadecane is ~10 kcal/mol, whereas the gap between cyclohexadecane and cyclopentadecane is ~5 kcal/mol. It is likely that the heat of formation of cycloheptadecane is too negative. A value of -83.0 kcal/mol for the heat of formation would return a total positive strain energy of 1.7 kcal/mol. Indeed, the heat of formation of cyclotetradecane was corrected in 1992 from a former value of -57.2 kcal/mol which had given a higher total strain of +12.6 kcal/mol. Of course, the issue of cycloheptadecane could have been dropped from the Table 1 and the graph and you would have been none the wiser!