
Hess's Law (G. H. Hess, 1840) or the Law of Constant Heat Summation states that the heat absorbed or liberated by any chemical reaction is independent of path. Thus, if A is converted to D directly or the reaction follows the path A --> B --> C --> D, the total heat of the reaction (enthalpy, ΔHo) is the same. In the latter instance, the sum of the heats of the individual reactions A --> B, B --> C, and C --> D equals the heat of reaction for A --> D. These reactions are generally considered to occur at 25 oC and 1 atm. Under these conditions, for example, H2, O2, N2, Cl2, CO2, and methane (CH4) are gases; water, Br2, hexanes (C6H14) are liquids; and carbon (graphite, diamond, buckyball) and I2 are solids. Thus, the following relationship exists:
ΔHo(rxn) = ΔHo
(products) - ΔHo(reactants)
The
Combustion of Carbon and Hydrogen
Consider the following reactions: [(g) = gas, (l) = liquid, (s) = solid]
1) C(s) + O2(g) -------> CO2(g) ΔHo = -94.05 kcal/mol
2a) H2(g) + 1/2 O2(g) --------> H2O(l) ΔHo = -68.3 kcal/mol
2b) H2O(l) ----------> H2O(g) ΔHvo = +10.5 kcal/mol
2c) H2(g) + 1/2 O2(g) --------> H2O(g) ΔHo = -57.8 kcal/mol
In reaction 1, carbon in the form of graphite, the most stable
allotropic form of carbon, is oxidized to CO2 liberating
94.05 kcal/mol of heat. These experiments can be conducted in a bomb
calorimeter. The negative value indicates that the reaction is
exothermic (think downhill). In reaction 2a, the oxidation, i.e.,
combustion, of hydrogen forms water exothermically as a liquid at 25
oC. If we add equation 2b, the heat of vaporization (ΔHvo)
of water, and equation 2a, then equation 2c reflects the formation of
water as a gas from the combustion of hydrogen at 25 oC
and 1 atm.
If ΔHo(reactants) were to have a value
of zero in eq. 1, 2a, and 2c, then ΔHo(rxn)
would equal ΔHo (products). In other
words, we would have determined the heats of formation ( ΔHfo)
of CO2 and H2O, which, in this instance, are
also the heats of combustion of carbon and hydrogen, respectively.
Why do the reactants in these reactions have a value of zero? Because
they are assigned that value! The elements in their most stable
states have ΔHfo = 0: C(s, graphite),
H2(g), O2 (g), N2 (g), Cl2
(g), Br2 (l) and I2 (s). In the heats
of formation we will be using, bromine (ΔHfo
= ΔHvo) and iodine (ΔHfo
= ΔHosublimation) are treated as gases as
is water at 25 oC and 1 atm..
The
Combustion of Methane at 25 oC
Methane ( CH4) undergoes combustion according to the balanced equation shown below:
CH4(g)+ 2O2 (g) -------> CO2 (g) + 2H2O (l); ΔHo(rxn) = -212.8 kcal/mol
For this reaction,
ΔHo(rxn) = ΔHfo (CO2) + ΔHfo (2 x H2O) - ΔHfo(CH4) - ΔHfo(O2)
-212.8 = -94.05 + 2(-68.3) - ΔHfo(CH4) - 0
From the solution of this equation, the heat of formation of methane can be determined as -17.9 kcal/mol. Although we can write for the formation of methane from carbon and hydrogen
C(s) + 2H2(g) ------> CH4(g)
the heat of this reaction cannot be determined directly. The value is determined by the difference of three measureable quantities: The heats of combustion of CH4, carbon (graphite), and hydrogen. The reaction can be visualized as follows:

By this difference technique, many heats of formation of compounds
have been determined. Look
here.
Heats
of Hydrogenation
Heats of hydrogenation may be determined experimentally or calculated using heats of formation. Consider the heat of hydrogenation of the two isomers of 2-butene to n-butane. Look up the heats of formation of n-butane, (Z)-2-butene, and (E)-2-butene. Our basic equation is:
ΔHo(rxn) = ΔHo (products) - ΔHo(reactants)
So that for (Z)-2-butene
ΔHo(H2) = ΔHo(n-butane) - ΔHo((Z)-2-butene) -------------------> ΔHo(H2) = (-30.0) - (-1.7) = -28.3 kcal/mol
and for (E)-2-butene
ΔHo(H2) = ΔHo(n-butane) - ΔHo((E)-2-butene) -------------------> ΔHo(H2) = (-30.0) - (-2.7) = -27.3 kcal/mol
The hydrogenation of the two alkenes is exothermic and the
difference in the heats of hydrogenation reflects the difference in
the heats of formation of the two alkenes. (Z)-2-Butene, which has a
less negative heat of formation than (E)-2-butene, is destabilized by
1.0 kcal/mol.
Bond
Dissociation Energies (DHo)
The bond dissociation energy (BDE) is the energy required to break a bond homolytically into its two radicals. Bonds that are broken are endothermic (+DHo); bonds that are formed are exothermic (-DHo).
Is there a relationship between the heats of formation of compounds and radicals, and bond dissociation energies? In the diagram below, methane is formed from carbon and hydrogen in their standard states [ΔHfo (CH4)]. The heat of formation of methane is exothermic. When a C-H bond in methane is dissociated homolytically (BDE), the total energy expended is for the formation of a methyl radical and a hydrogen atom. The algebraic sum of these two processes is equal to the heat of formation of a methyl radical and a hydrogen atom derived from carbon and hydrogen in their standard states. Even though the absolute sum of the lengths of the arrows for DHo (CH4) plus ΔHfo (CH4) is greater than the other pair, the ΔHfo (CH4) is a "negative arrow". Imagine that the ΔHfo of some substance were positive, e.g., the alkyne acetylene, the arrow would be "positive" and point upward from the standard state.
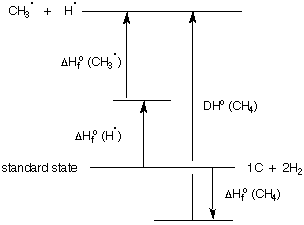
Therefore, for some generalized structure such as hydrocarbon RH,
ΔHfo (RH) + DHo (RH) = ΔHfo (R.) + ΔHfo (H.)
Given any three values, the fourth one can be calculated.