Average
Bond Energies
Heats of
Atomization
As a prelude to this topic, you should be
familiar with Hess's
Law, Heats
of Formation, Heats
of Combustion and Bond
Dissociation Energies (BDE's).
|
Index:
|
Some
Relevant Atoms and Radicals
|
ΔHfo
(kcal/mol)
|
|
H
|
+52 (1/2 BDE)
|
|
Cl
|
+29 (1/2 BDE)
|
|
Br
|
+23 (1/2 BDE)
|
|
I
|
+18 (1/2 BDE)
|
|
C
|
+171
|
|
CH3 (see
below)
|
+34
|
|
C2H5 (see
below)
|
+26
|
|
O
|
+59.6
|
|
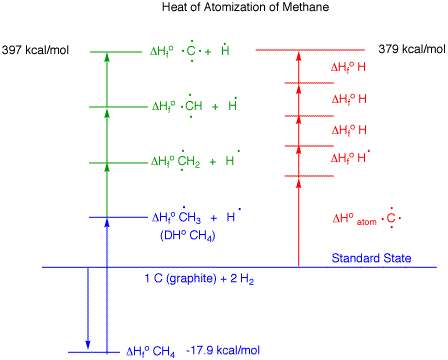
|
|
C-H
Bonds:
Average Bond Energies (ABE's)
provide a means of estimating the strength of bonds such as
C-H, C-O, carbonyls, double bonds and triple bonds. The
method employs heats of atomization and heats of formation.
The ABE's are calculated averages and not directly
measurable quantities like bond dissociaton energies
(BDE's). The table above lists the heats of formation of
atoms and radicals that have been imported from the
BDE
Tables. The diagram on the upper
right, which is not to scale, describes the heat of
atomization of methane. The section in blue describes the
type of diagram employed in illustrating bond
dissociation energy (BDE) of
methane. Rather than stop at the
formation of a methyl radical and a hydrogen atom, what if
C-H bonds were successively cleaved from the methyl radical
to afford CH2, CH and C along with three atoms of
hydrogen (in
green). The sum total of the blue and
green areas equals what is in red. The calculated heat of
atomization of 1 mole of graphite and the formation of four
moles of hydrogen atoms is 379 kcal/mol. From this total is
subtracted the negative heat of formation of methane to give
a value of 397 kcal/mol for the heat of atomization of
methane. Of course, methane has four C-H bonds. If the heat
of atomization of methane is divided by four, the average
bond energy (ABE) for a C-H bond is 99 kcal/mol. Just as a
class average grade may be 85 (we certainly hope so) no
single grade need be 85. Likewise, no C-H bond need be 99
kcal/mol. Indeed, with an average of 99 kcal/mol and an
initial bond dissociation energy of 104 kcal/mol, at least
one of the successive processes
(in
green) must be less than 99 kcal/mol.
The energy for each of these steps is known. Using a more
up-to-date BDE (105 kcal/mol) for methane, Ellison
has reported the following values
(Table 1) for the stepwise conversion CH4
-----> C + 4H. Notice that three of the BDE's are greater
than the ABE while one is smaller.
The same ABE can be obtained using the heat of formation of
methane in conjunction with the heats of formation of carbon
and hydrogen atoms (Table 2).
|
Table 1
|
|
|
|
BDE (kcal/mol)
|
ABE (C-H)
|
|
CH4
------->
|
CH3
|
H
|
105
|
|
|
CH3
------->
|
CH2
|
H
|
110
|
|
|
CH2
------->
|
CH
|
H
|
101
|
|
|
CH ------->
|
C
|
H
|
81
|
|
|
SUM: CH4
------->
|
C
|
4H
|
397
|
99
|
Table 2
|
ΔHo/rxn.--->
|
CH4
|
------>
|
C
|
4H
|
|
ΔHfo
|
-17.9
|
------>
|
+171
|
4 (+52)
|
|
ΔHrxno
|
+397
|
|
|
|
|
ΔHABEo/
C-H bond
|
+99
|
|
|
|
|
|
C-C
Single Bonds:
What is an ABE for a C-C
single bond? This value is readily obtained by analyzing
alkanes such as ethane, which has only C-H bonds and a
single C-C single bond (Table 3). Ethane upon "atomization"
yields two carbon atoms and six hydrogens for a total of 654
kcal/mol. Subtracting -20 kcal/mol for the ΔHfo
of ethane leaves 674 kcal/mol for this hypothetical
reaction. Subtraction of 594 kcal/mol for the six hydrogen
atoms leaves 80 kcal/mol for the ABE of a C-C single bond.
Higher homologs of ethane, ---namely -- propane, n-butane,
isobutane and cyclohexane give values of 81, 81, 82 and 82
kcal/mol, respectively. In each of these examples, one must
divide the "final" total by the number of C-C bonds.
Clearly, a strained ring system would not be able to provide
an accurate ABE for the C-C bond. For cyclopropane the ABE
is 72.5 kcal/mol. Because ethane is less representative of
alkanes than alkanes with methylene and methine hydrogens,
ethane is atypical. For unstrained systems, 82 kcal/mol is
the ABE for a C-C single bond.
|
Table 3
|
|
CH3CH3
|
------>
|
2C
|
6H
|
|
ΔHfo
|
-20
|
------>
|
2(+171)
|
6(+52)
|
|
ΔHrxno
|
+674
|
|
|
|
|
ΔHABEo
C-H
|
6(+99) = 594
|
|
|
|
|
ΔHABEo
/C-C
|
674-594 =
80
|
|
|
|
|
|
C=C
Double Bonds:
Now consider the strength of
a C=C double bond. Ethylene serves as a useful example. The
computation in Table 4 reveals an ABE for the C=C double
bond of 142 kcal/mol. But ethylene is not typical of most
double bonds since it is one of a kind. Compare some of the
constitutional isomers of the hexenes: (1-hexene, 144
kcal/mol; (E)-3-hexene, 144 kcal/mol;
(E)-3-methyl-2-pentene, 149 kcal/mol; 2,3-dimethyl-2-butene,
151 kcal/mol. As the double bond becomes more substituted
with carbon atoms, the double bond becomes more stable. This
ordering of energies is also reflected in the heats of
formation. An ABE for a C=C double bond is ~146
kcal/mol.
Since the double bond has, in addition to a σ-bond, it
also has a π-bond. Not surprisingly, double bonds are
stronger than C-C single bonds. How much stronger? Take the
difference to find the contribution of a π-bond. Thus,
146 - 82 = 64 kcal/mol for a π-bond.
|
Table 4
|
|
CH2=CH2
|
------>
|
2C
|
4H
|
|
ΔHfo
|
+12.5
|
------>
|
2(+171)
|
4(+52)
|
|
ΔHrxno
|
+538
|
|
|
|
|
ΔHABEo/C-H
|
4(+99) = 396
|
|
|
|
|
ΔHABEo/C=C
|
538-396 =
142
|
|
|
|
|
|
CC
Triple Bonds:
The CC triple bond is
stronger than the C=C double bond. See acetylene in (Table
5). As with the case of the double bond, increased carbon
substitution increases the stability of the triple bond.
Accordingly, 1-butyne and 1-hexyne have a triple bond ABE of
199 kcal/mol while the internal alkynes 2-butyne, 2- and
3-hexyne have ABE's of 203 kcal/mol. Employing an average
ABE of 201 kcal/mol for a triple bond, the incremental π-bond
is calculated to be worth 55 kcal/mol (201 - 146 kcal/mol).
The second π-bond in an alkyne is ~9 kcal/mol less stable
than the π-bond in an alkene.
|
Table 5
|
|
HCCH
|
------>
|
2C
|
2H
|
|
ΔHfo
|
+54.5
|
------>
|
2(+171)
|
2(+52)
|
|
ΔHrxno
|
+392
|
|
|
|
|
ΔHABEo
C-H
|
2(+99) = 198
|
|
|
|
|
ΔHABEo
/CC
|
392-198 =
194
|
|
|
|
|
|
C-O
Bonds:
The C-O single bond ABE can
be determined as shown in Tables 6 and 7 using dimethyl
ether and diethyl ether as models. As was the case with
using ethane as a model, dimethyl ether gives a value for
the C-O ABE of 82 kcal/mol. On the other hand, diethyl ether
affords an ABE of 85 kcal/mol. Based upon several
calculations. an "average" ABE for the C-O bond is 84
kcal/mol.
|
Table 6
|
|
CH3OCH3
|
------>
|
2C
|
6H
|
O
|
|
ΔHfo
|
-44.0
|
------>
|
2(+171)
|
6(+52)
|
+59.6
|
|
ΔHrxno
|
+758
|
|
+342
|
+312
|
+59.6
|
|
ΔHABEo
C-H
|
6(+99) = 594
|
|
|
|
|
|
ΔHABEo/C-O
|
(758-594)/2 =
82
|
|
|
|
|
Table 7
|
|
CH3CH2OCH2CH3
|
------>
|
4C
|
10H
|
O
|
|
ΔHfo
|
-60.4
|
------>
|
4(+171)
|
10(+52)
|
+59.6
|
|
ΔHrxno
|
+1324
|
|
684
|
+520
|
+59.6
|
|
ΔHABEo
C-H
|
10(+99) = 990
|
|
|
|
|
|
ΔHABEo
C-C
|
2(+82) = 164
|
|
|
|
|
|
ΔHABEo
/C-O
|
(1324-990-164)/2 =
85
|
|
|
|
|
|
|
O-H
Bonds:
The heat of formation of an oxygen
atom is ~60 kcal/mol. At 25oC water is a liquid.
The O-H bond in water has an ABE of 116 kcal/mol (Table 8).
Its heat of formation is -68.3 kcal/mol. As a gas at this
temperature, the heat of vaporization drops the heat of
formation to -57.8 kcal/mol and, accordingly, the bond
strength is 111 kcal/mol for water in the gas phase.
Primary alcohols [ethanol (Table 9), 1-propanol]
have ABE's of 109 kcal/mol; secondary alcohols (2-propanol,
2-butanol) have values of 113 kcal/mol; and tertiary
alcohols (t-butanol) an ABE of 117 kcal/mol. Ethylene
glycol, which has two primary hydroxyls, has an O-H bond ABE
of 111 kcal/mol. An ABE of 111 kcal/mol is a convenient
estimate for the ABE of the O-H bond.
|
Table 8
|
|
H2O (l)
|
H2O (g)
|
------>
|
2H
|
O
|
|
ΔHfo
|
-68.3
|
-57.8
|
------>
|
2(+52)
|
+59.6
|
|
ΔHrxno
|
+232
|
+222
|
|
+104
|
+59.6
|
|
ΔHABEo
/O-H bond
|
+116
|
+111
|
|
|
|
Table 9
|
|
CH3CH2OH
|
------>
|
2C
|
10H
|
O
|
|
ΔHfo
|
-56.2
|
------>
|
2(+171)
|
6(+52)
|
+59.6
|
|
ΔHrxno
|
+770
|
|
+342
|
+312
|
+59.6
|
|
ΔHABEo
C-H
|
5(+99) = 495
|
|
|
|
|
|
ΔHABEo
C-C
|
+82
|
|
|
|
|
|
ΔHABEo
/C-O
|
+84
|
|
|
|
|
|
|
770-495-82-84 =
109
|
|
|
|
|
|
|
C=O
Bonds: Aldehydes and Ketones
The calculation of the ABE of
a carbonyl group (C=O) follows the same method that was
employed in computing the ABE of a C=C double bond. As was
the case with C=C double bonds, there is a variation of the
ABE that depends upon what atoms are attached to the carbon
of the carbonyl. Like ethylene, formaldehyde (Table 10)
gives a lower value than typical aldehydes (Table 12). For
ketones the ABE is typically in the range 176 - 179 kcal/mol
(Table 11). Cyclopentanone and cyclopentanone both have
ABE's of 176 kcal/mol.
Table 11
|
|
(CH3)2C=O
|
------>
|
3C
|
6H
|
O
|
|
ΔHfo
|
-52.2
|
------>
|
3(+171)
|
6(+52)
|
+59.6
|
|
ΔHrxno
|
+937
|
|
|
|
|
|
ΔHABEo
C-H
|
6(+99) = 594
|
|
|
|
|
|
ΔHABEo
C-C
|
2(+82) = 160
|
|
|
|
|
|
ΔHABEo
/C=O
|
937-594-164 =
179
|
|
|
|
|
|
Table 10
|
|
CH2=O
|
------>
|
C
|
2H
|
O
|
|
ΔHfo
|
-27.7
|
------>
|
(+171)
|
2(+52)
|
+59.6
|
|
ΔHrxno
|
+362
|
|
|
|
|
|
ΔHABEo
C-H
|
2(+99) = 198
|
|
|
|
|
|
ΔHABEo
C=O
|
362-198 =
164
|
|
|
|
|
Table 12
|
|
CH3CHO
|
------>
|
2C
|
4H
|
O
|
|
ΔHfo
|
-40.8
|
------>
|
2(+171)
|
4(+52)
|
+59.6
|
|
ΔHrxno
|
+650
|
|
+342
|
+208
|
+59.6
|
|
ΔHABEo
C-H
|
4(+99) = 396
|
|
|
|
|
|
ΔHABEo
C-C
|
+82
|
|
|
|
|
|
ΔHABEo/C=O
|
650-396-82 =
172
|
|
|
|
|
|
|
C=O
Bonds: Carboxylic Acids
The carbonyl group of
carboxylic acids has an ABE of 199 kcal/mol. Acetic acid
(Table 14) is typical. Formic acid, like formaldehyde (Table
13) compared to other aldehydes, has a lower carbonyl ABE
(181 kcal/mol).
|
Table 13
|
|
HCO2H
|
------>
|
1C
|
2H
|
2O
|
|
ΔHfo
|
-80.5
|
------>
|
+171
|
2(+52)
|
2(+59.6)
|
|
ΔHrxno
|
+475
|
|
+171
|
+104
|
+119.2
|
|
ΔHABEo
C-H
|
+99
|
|
|
|
|
|
ΔHABEo
C-O
|
+84
|
|
|
|
|
|
ΔHABEo
O-H
|
+111
|
|
|
|
|
|
ΔHABEo
/C=O
|
475-99-84-111 =
181
|
|
|
|
|
Table 14
|
|
CH3CO2H
|
------>
|
2C
|
4H
|
2O
|
|
ΔHfo
|
-103.5
|
------>
|
2(+171)
|
4(+52)
|
2(+59.6)
|
|
ΔHrxno
|
+773
|
|
+342
|
+208
|
+119.2
|
|
ΔHABEo
C-H
|
3(+99) = 297
|
|
|
|
|
|
ΔHABEo
C-C
|
+82
|
|
|
|
|
|
ΔHABEo
C-O
|
+84
|
|
|
|
|
|
ΔHABEo
O-H
|
+111
|
|
|
|
|
|
ΔHABEo
/C=O
|
773-297-82--84-111 =
199
|
|
|
|
|
|
|
C=O
Bonds: Esters and Carbonates
The ABE of the carbonyl group
of esters are comparable to the values determined for
carboxylic acids. Formates (Table 16) are typically lower
valued than esters of other carboxylic acids. The 202
kcal/mol for the carbonyl ABE of ethyl acetate (Table 17) is
what is seen with most aliphatic esters. Note the
progression of carbonyl bond strength in the series:
formaldehyde, aldehyde, ketone, ester and carbonate (Table
16, 216 kcal/mol). Successive interpolation of carbon and
oxygen increases the ABE of the carbonyl group.
Table 16
|
|
(C2H5O)2CO
|
------>
|
5C
|
10H
|
3O
|
|
ΔHfo
|
-152.5
|
------>
|
5(+171)
|
10(+52)
|
3(+59.6)
|
|
ΔHrxno
|
+1031
|
|
+855
|
+520
|
+178.8
|
|
ΔHABEo
C-H
|
10(+99) = 990
|
|
|
|
|
|
ΔHABEo
C-C
|
2(+82) = 164
|
|
|
|
|
|
ΔHABEo
C-O
|
2(+84) = 168
|
|
|
|
|
|
ΔHABEo
/C=O
|
1031-990-164-168 =
216
|
|
|
|
|
|
Table 15
|
|
HCO2CH2CH3
|
------>
|
3C
|
6H
|
2O
|
|
ΔHfo
|
-86.5
|
------>
|
3(+171)
|
6(+52)
|
2(+59.6)
|
|
ΔHrxno
|
+1031
|
|
+513
|
+312
|
+119.2
|
|
ΔHABEo
C-H
|
6(+99) = 594
|
|
|
|
|
|
ΔHABEo
C-C
|
+82
|
|
|
|
|
|
ΔHABEo
C-O
|
2(+84) = 168
|
|
|
|
|
|
ΔHABEo
/C=O
|
1031-594-82-168 =
187
|
|
|
|
|
Table 17
|
|
CH3CO2CH2CH3
|
------>
|
4C
|
8H
|
2O
|
|
ΔHfo
|
-106.5
|
------>
|
4(+171)
|
8(+52)
|
2(+59.6)
|
|
ΔHrxno
|
+1326
|
|
+684
|
+416
|
+119.2
|
|
ΔHABEo
C-H
|
8(+99) = 792
|
|
|
|
|
|
ΔHABEo
C-C
|
2(+82) = 164
|
|
|
|
|
|
ΔHABEo
C-O
|
2(+84) = 168
|
|
|
|
|
|
ΔHABEo
/C=O
|
1326-792-164-168 =
202
|
|
|
|
|
|
|
C-X
Bonds: Halides
The calculation of ABE's for
C-X bonds of halides are in the range of BDE's but there is
a fairly wide range of values. The variance in BDE's for
C-Cl bonds (Table 18) is small and it is a value of 81
kcal/mol that is often employed in computations. The same
situation applies to alkyl bromides. A bond dissociation
energy of 68 kcal/mol is utilized for the ABE. This makes
sense since the BDE is an experimental value with a small
range of values for the BDE.
Table 18
|
RCl (kcal/mol)
|
ABE
|
BDE
|
|
chloromethane
|
79
|
84
|
|
chloroethane
|
81
|
81
|
|
1-chloropropane
|
81
|
81
|
|
1-chlorobutane
|
81
|
81
|
|
2-chloropropane
|
84
|
80
|
|
2-chlorobutane
|
84
|
80
|
|
2-chloro-2-methylpropane
|
87
|
79
|
|
Table 19
|
RBr (kcal/mol)
|
ABE
|
BDE
|
|
bromomethane
|
61
|
70
|
|
bromoethane
|
63
|
68
|
|
1-bromopropane
|
64
|
68
|
|
1-bromobutane
|
64
|
68
|
|
2-bromopropane
|
67
|
68
|
|
2-bromobutane
|
67
|
68
|
|
2-bromo-2-methylpropane
|
70
|
65
|
|
Return to
the Top of the Page
