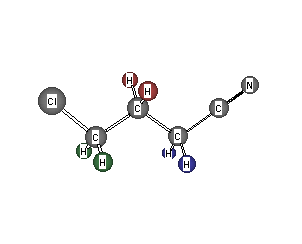
Formula: C4H6ClN
Answer: 4-Chlorobutyronitrile
Chemical Shift Assignments: δ 1.98 (pentuplet, 2H), 2.43 (triplet, 2H), 3.51 (triplet, 2H)
The formula indicates two degrees of unsaturation. The integration must be doubled to accommodate the six hydrogens of the formula. This pattern looks similar to the previous problem. The pattern indicates -CH2CH2CH2-, which leaves CClN. Chlorine must be on one terminus and the other end must be a cyano group (RCN), or its isomeric structure, an isonitrile (RNC). You haven't learned about isonitriles. A -CH2- next to a cyano group is not in the chemical shift chart but you can compare the chemical shift of the methylene hydrogens with those in n-butyronitrile here (Enter n-butyronitrile as the compound name).
The 13C spectrum displays four singlets. The one at 120.4 ppm is of weak intensity (no hydrogens attached) and belongs to the carbon of the nitrile (-CN). The signal at 44.6 ppm is the -CH2Cl while the remaining carbons are at 29.5 ppm (-CH2CN) and 15.9 ppm (-CH2CH2CH2-). Return to Menu.
 |
chlorine - light green |
|---|