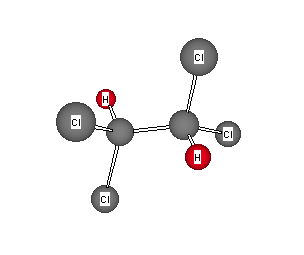
Formula: C2H2Cl4
Answer: 1,1,2,2-Tetrachloroethane
The formula indicates no degrees of unsaturation. The presence of only one singlet in the 1H (δ 5.97) and 13C (74.41 ppm) NMR indicates that there are two equivalent hydrogens and two equivalent carbons. There are only two ways to attach two hydrogens and four chlorines to two carbons. Clearly, 1,1,1,3-tetrachloroethane would have a singlet in its proton spectrum but two singlets in its carbon spectrum. The choice must be the symmetrical 1,1,2,2-isomer. Return to Menu.