Formula: C7H8O
Answer: Benzyl Alcohol
Chemical Shift Assignments: 1H NMR: δ 2.91 (1H, t, J = 5.3 Hz), 7.33 (3H, d, J = 5.3 Hz), and 7.25 - 7.42 (5H, m)
The degree of unsaturation is 4. The five aromatic hydrogens have virtually the same chemical shift, which creates a complex signal. This pattern is typical of aromatic rings mono-substituted with an sp3 carbon, e. .g, toluene. Note the difference in the preceding two problems (#6 and #7). After subtracting the phenyl (C6H5-) from the structure, CH3O- remains. Because their are two signals for which to account, a methoxy group is excluded (no 3H singlet) and -CH2OH is the only option. The coupling indicates that the OH is not exchanging rapidly on the NMR time scale. If rapid exchange were to occur --- say, in the presence of an acid --- the signals at δ 2.91 and 7.33 would be singlets.
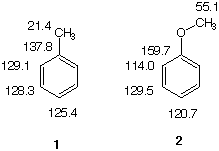
The 13C NMR spectrum reveals five of the seven carbon atoms. There are two identical ortho and meta carbons. One carbon signal is located at 63.8 ppm (-CH2O-) and the remaining four are in the aromatic carbon region of the spectrum. The appearance of four aromatic carbons suggests a monosubstituted (one carbon) benzene ring (six carbons). That the attachment of the substituent to the benzene ring is -CH2OH and is not -OCH3 -- apart from the 1H NMR evidence above --, is revealed in a comparison of the shifts of the aromatic carbons in toluene 1 (carbon substituent) and anisole 2 (oxygen substituent) is informative (see below). The chemical shifts of the aromatic carbons in benzyl alcohol are closer to the aromatic carbon shifts in toluene than they are to those in anisole. Moreover, the stronger ortho, para-director oxygen shields the ortho and para positions in anisole to a greater extent than the ortho and para positions in toluene. The weak signal at 140.6 ppm is the ipso carbon while the weakest of the remaining aromatic signals (126.9 ppm) is the carbon at the para position. Given the inductive effect of the substituent to provide electron density (shielding, upfield shift) at the ortho and para positions, the signal at 126.6 ppm is assigned to the carbons at the ortho position. The meta position carbons are at 128.0 ppm. Return to Menu.
 |
oxygen-yellow
|
 |
|---|