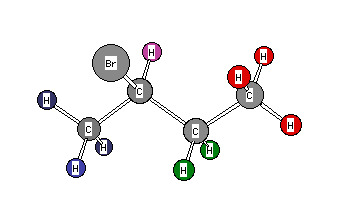
Formula: C4H9Br
Answer: 2-Bromobutane (sec-Butyl bromide)
Chemical Shift Assignments: δ 1.03(t, 3H), 1.71 (d, 3H), 1.82 (2H, pentuplet), and 4.12 (1H, sextet)
The degree of unsaturation is zero. The compound must be one of the four possible butyl bromides. Two non-equivalent 3H signals, one a triplet (δ 1.03) and the other a doublet (δ 1.71) can only mean 2-bromobutane. The methine hydrogen on the carbon bearing the bromine ( δ 4.12) is coupled to five equivalent vicinal protons. However, contrary to the diastereotopic hydrogens in the preceding problem, the diastereotopic methylene hydrogens in 2-bromobutane fortuitously have the same chemical shift ( δ 1.82). They do not couple with one another, there by simplifying this signal. They are coupled to four vicinal hydrogens to give a pentuplet. Return to Menu.