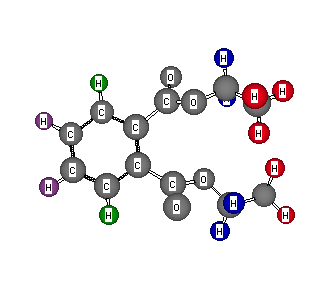
Formula: C12H14O4
Answer: Diethyl phthalate
Chemical Shift Assignments:
1H NMR: δ 7.61 (1H, m), 7.28 (1H, m), 4.15 (2H, quartet, J = 7 Hz), and 1.13 (3H, t, J = 7 Hz)
13C NMR: 167.1, 132.0, 130.6, 128.5, 61.3, and 13.6 ppm.
The degree of unsaturation is 6. The 1H NMR spectrum accounts for 7 of 14 hydrogens while the 13C spectrum indicates 6 of 12 carbons. Clearly, there is symmetry in the compound. The signals in the 1H NMR spectrum at δ 4.15 and 1.13 constitute to equivalent ethyl patterns. The first signal indicates attachment to an oxygen, thus 2 x CH3CH2O-, leaving C8H4O2. The presence of the signal at 167.1 ppm in the 13C NMR suggests an ester carbonyl. Now we have 2 x CH3CH2OC=O, leaving C6H4, and 4 degrees of unsaturation for which to account. The signals at δ 7.61 and 7.28 are aromatic hydrogens (2 of each). The compound cannot be para-substituted (1 aromatic hydrogen) nor meta-substituted (3 aromatic hydrogens). Thus it must be ortho-substituted. Something should be bothering you! Would you not expect a simple doublet for each aromatic hydrogen rather than the complex patterns observed? In all the examples in the Novice Level and all the preceding examples in the Intermediate Level, all equivalent hydrogens have been chemically and magnetically equivalent. Here each aromatic hydrogen is chemically equivalent but magnetically non-equivalent. This phenomenon occurs with identical pairs of hydrogens within coupling distance of each other. Imagine all four of the aromatic hydrogens having β-spins. Then each type (green or purple) of hydrogen experiences the same field. But if one purple hydrogen had an α-spin, each of the green hydrogens would experience different magnetic fields, therefore splitting. The same phenomenon is seen with the two 2,3-dibromobutanes. Return to Menu.
 |
oxygen-yellow
|
|---|