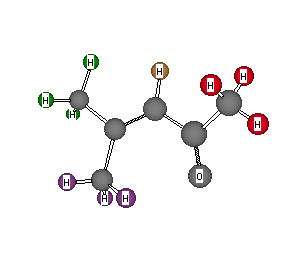
Formula: C6H10O
Answer: 4-Methyl-3-penten-2-one
(Mesityl oxide)
Chemical Shift Assignments:
1H NMR: δ 5.72 (1H, brd. s), 1.75 (3H, s), 1.74 (3H, s) and 1.49 (3H, s)
13C NMR: 197.48, 154.12, 123.70, 30.98, 26.85, 19.88 ppm.
The degree of unsaturation is 2. The weak singlet at 197.48 ppm indicates a carbonyl group of a ketone. If you check the 13C shift table, you will see that amides and esters, which have similar chemical shifts, are excluded by the formula. The carbon is not that of an aldehyde because there is no low field aldehyde hydrogen in the 1H NMR. Removing C=O from the formula gives C5H10. The 1H NMR reveals three methyl singlets, two of which are quite similar (δ 1.74 and 1.75 ). Subtracting 3 x CH3 from the residual formula gives C2H, which must contain 1 degree of unsaturation. That is, C2H is C=CH to which three methyls (uncoupled) and C=O must be attached. The solution below is the only possibility. If one of the methyl groups attached to the double bond were switched with the vinyl hydrogen, then the hydrogen and the remaining methyl group would be coupled to one another. Return to Menu.
 |
oxygen-yellow
|
|---|