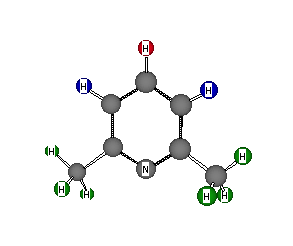
Formula: C7H9N
Answer: 2,6-Dimethylpyridine
(2,6-lutidine; a lutidine is a dimethylpyridine)
Chemical Shift Assignments:
1H NMR: δ 6.75 (1H, t, J = 8.0 Hz), 6.25 (2H, d, J = 8.0 Hz), and 1.87 (6H, s)
13C NMR: 156.56, 135.33, 119.01, and 23.48 ppm.
The degree of unsaturation is 4. The 13C NMR spectrum contains only four types of carbon, yet there are seven carbons in the molecule. This indicates some symmetry element. Three pairs of equivalent carbons and one more equals seven. With only one nitrogen, it must lie in the symmetry element ( a plane?) along with the odd carbon. The low field 1H NMR signals indicate that they are on an aromatic ring and that they are on three contiguous carbons with the central hydrogen coupled by J = 8 Hz (ortho coupling) to both of the adjacent neighbors. These data suggest a pyridine ring with methyl groups at the 2,6-positions rather than at positions 3 and 5. If the latter were the case, the value for J would be much smaller. The assignment of the 13C signals is of interest. Obviously, the high field singlet at 23.48 ppm is the methyl groups. Of the aromatic carbons, the signal at 119.01 ppm is the C3 and C5 carbons. It is the most intense signal of the three, there are two of them, and they have hydrogens attached (blue) to the them, which enhances relaxation and intensifies the signal. The weakest signal of the three is at 156.56 ppm. Is this signal the carbons at C2 and C6 (two of them but no hydrogens) or the carbon at C4 (one carbon with a hydrogen attached)? Tough question! The deshielding effect of the nitrogen is greatest on the proximate carbons. C2 and C6 are at 156.56 ppm. Assign the 13C signals in the previous problem. Return to Menu.
 |
nitrogen-light blue
|
|---|