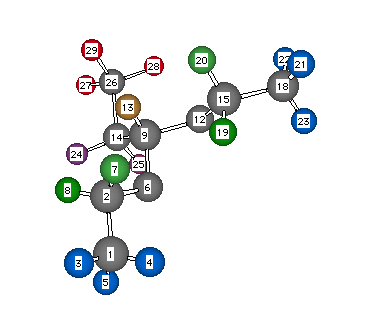
Formula: C7H16O2
Answer: Propionaldehyde, diethyl acetal
Chemical Shift Assignments: 1H NMR: δ 0.63 (3H, t, J = 8 Hz), 0.92 (6H, t, J = 7 Hz), 1.34 (2H, dq, J = 8 and 6 Hz), 3.20 (2H, dq, J = 7 and 9 Hz), 3.35 (2H, dq, J = 7 and 9 Hz), and 4.12 (1H, t, J = 6 Hz)
The degree of unsaturation is 0. Although this sample has some contamination, the impurities do not account for its complexity. This spectrum provides an example of diastereotopic protons. Let us first deal with the hydrogens on C9, C14, and C26 (see structure). The C26 CH3- group at δ 0.63 (integral = 3H) is a triplet because of coupling (J = 8 Hz) to the vicinal CH2 group. The same CH2 group (C14) causes the methine hydrogen H13 at δ 4.12 to appear as a triplet. The C14 methylene group is a doublet of quartets as a result of the unequal coupling to the C26 methyl group and the C9 methine hydrogen. The hydrogens H7 and H8 as well as H19 and H20 are diastereotopic. In the animation these diastereotopic hydrogens are in different shades of green. Hydrogens H7 and H19 are equivalent, as are H8 and H20. The diastereotopic hydrogens exist in different chemical and magnetic environments and therefore they have different chemical shifts. Thus, they couple to one another. They appear as a doublet of quartets, J = 7 and 9 Hz. Measure the coupling constants yourself. The two methyl groups at δ 0.92 are both triplets (J = 7 Hz), but the vicinal CH2 groups appear as separate signals at δ 3.20 and 3.35. The 13C NMR spectrum is simpler. A plane of symmetry in the molecule reduces the number of singlets to five: 103.81 (C9), 60.49 (C2, C15), 26.34 (C14), 14.99 (C1, C18), and 8.64 (C26) ppm. Note that the two oxygens attached to C9 deshield it relative to C2 and C15. Return to Menu.
 |
oxygen-yellow
|
|---|