Formula: C6H15PO3
Answer: Triethyl Phosphite
Chemical Shift Assignments: 1HNMR: δ 0.92 (3H, triplet) and 3.52 (2H, pentuplet)
The degree of unsaturation is 0. There are only 2 sets of signals integrating for a total of five hydrogens. Since the formula has 15 hydrogens, then the integration needs to be tripled. Clearly, there is a high level of symmetry in the molecule and the formula and symmetry dictate 3 x CH3CH2O-. But wait, this is no typical ethyl pattern! The upfield triplet (J = 8 Hz) looks fine but the CH2 group at δ 0.92 is a pentuplet (J = 8 Hz), not the expected quartet. Where is the fourth hydrogen? There isn't one! It is the phosphorus atom coupling through three bonds (vicinal coupling), just like a hydrogen atom. 31P couples (s = 1/2, natural abundance = 100%) to the CH2 group with a value of J the same as the vicinal H/H coupling constant.
The 13C spectrum displays two signals at: 16.36 and 57.16 ppm. Blow up these signals and you will see that they are doublets resulting from 13C/31P coupling. The carbon of the CH2 group at 57.16 ppm has the larger coupling (J = 11 Hz) while the more remote CH3 carbon at 16.36 ppm has J = 6 Hz. While there is broadband decoupling of the hydrogens, there is no decoupling of phosphorus. Return to Menu.
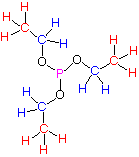 |
oxygen-yellow phosphorus-orange
|
|---|