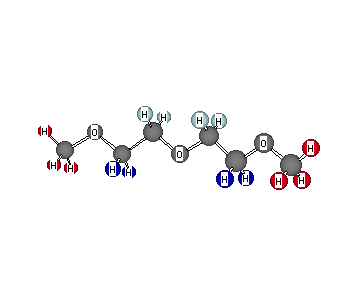
Formula: C6H14O3
Answer: Diethylene glycol, dimethyl ether (bis-2-Methoxyl ethyl ether)
Chemical Shift Assignments: 1H NMR: δ 3.00 (s,6H), 3.18 (m, 4H), and 3.27 (m, 4H)
The degree of unsaturation is 0. There are only 3 signals integrating for a total of seven hydrogens. Since the formula has 14 hydrogens, then the integration needs to be doubled. Clearly, there is a high level of symmetry in the molecule. The singlet at δ 3.00 is two methyl groups attached to oxygen [2 x CH3O-]. This leaves C4H8O, which, being symmetrically arranged to produce only two chemical shifts, leaves -CH2CH 2OCH2CH2-. These two signals are complex multiplets because they have chemical shifts that are close to one another. The value of Δδ is 0.09 ppm, which at 400 MHz, is 36 Hz. The expected pattern would be a pair of triplets with J = ~ 7- 8 Hz. Complex coupling arises when Δδ is close to the value of J. These hydrogens have been colored in different shades of blue to reflect their close chemical shifts.
The 13C spectrum displays singlets at: 58.07, 69.76, and 71.22. The 3 singlets reflects the symmetry in the molecule. The singlet at 58.07 ppm is the methyl carbons while the interior methylene carbons have been assigned to the singlet at 69.76 ppm. Return to Menu.
 |
oxygen-yellow
|
|---|