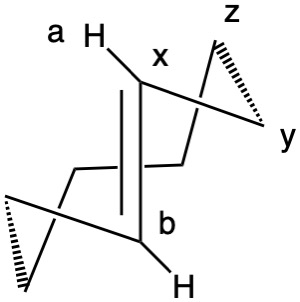
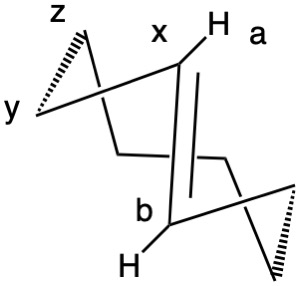
Planar chirality, like axial chirality, employs the descriptors P (plus) and M (minus). Chiral molecules having atoms a, b and x lying ina plane and y-z in a plane with atom z orthogonal to the plane fall into this category (Fig. 1). Implicit in this description is that z is restricted from lying in the plane. When b>a, the chirality is P (Rp, b>a>y>z).; when a>b, the assignment is M (Sp; a>b>y>z). Viewing down the y-x bond and b>a, P is set at 3 o'clock. When a>b, M is at 9 o'clock. To show that M = Sp, point your left (Sinister) thumb from y to x. Your fingers will point to atom a, the M-configuration. Conversely, your right (Rectus) hand will point to atom b, the P-configuration. [Note: JSmol is not able to assign planar configurations.]
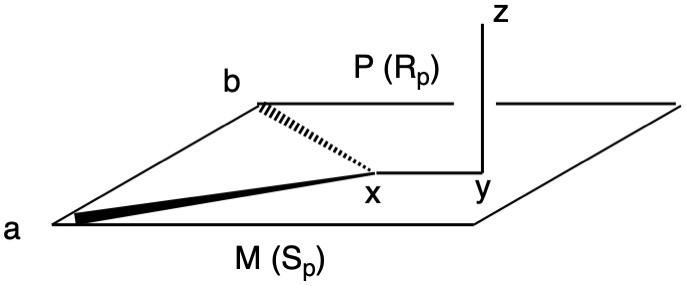 Fig. 1
Fig. 1