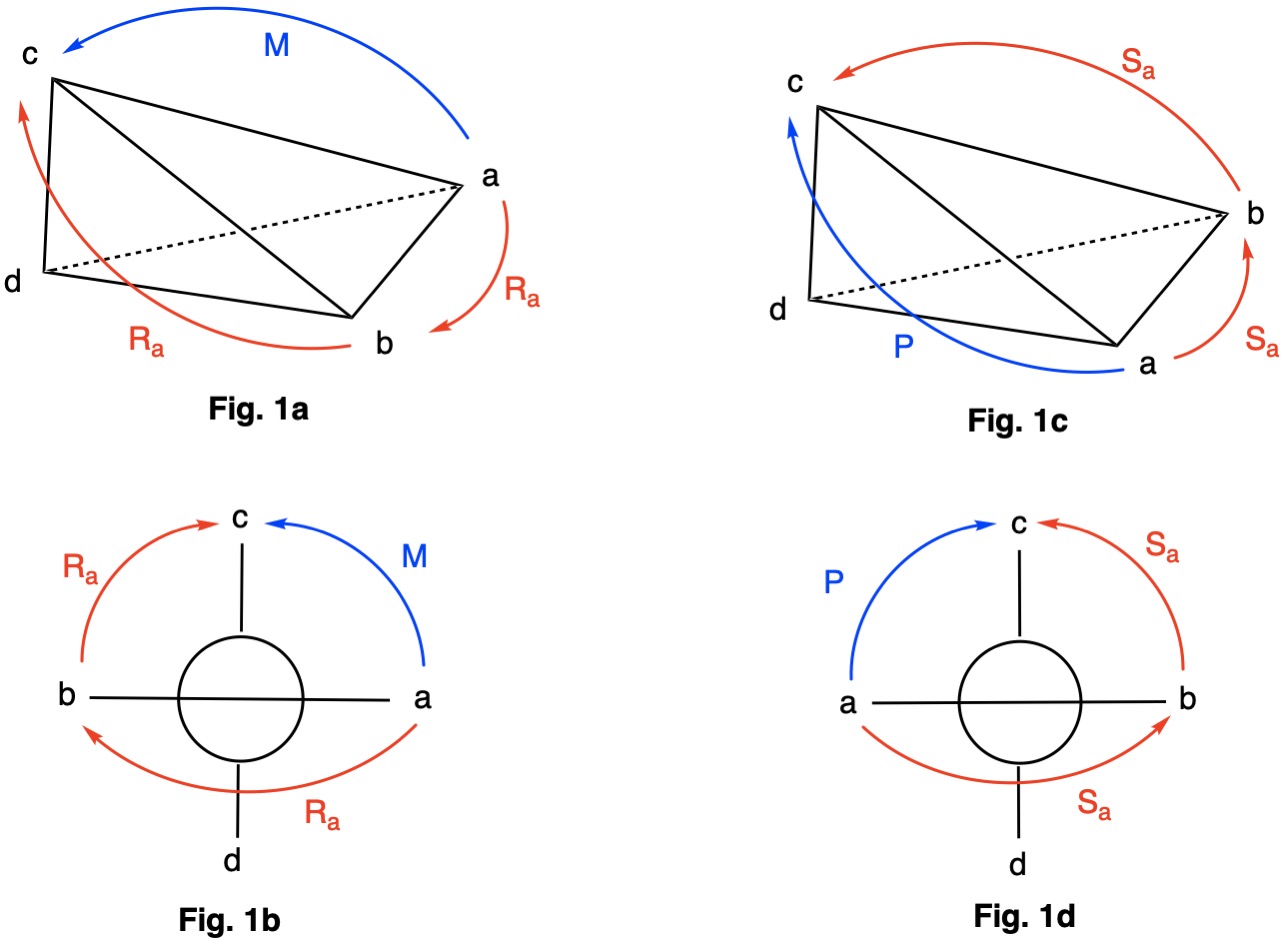
Axial chirality is generally applied to chiral allenes and biphenyls which lack the more common asymmetric carbon. The IUPAC 2013 BlueBook recommends the use of M (minus) and P (plus) descriptors. Older CIP (1966) rules utilize Ra and Sa , respectively. The priority orders are a>b>c>d. Figs. 1a & 1b illustrate the counterclockwise nature of the M-configuration and the right hand rule applied to the axial chirality Ra.
Figs. 1c & 1d illustrate the clockwise assignment of the P-configuration and the left hand rule applied to Sa.