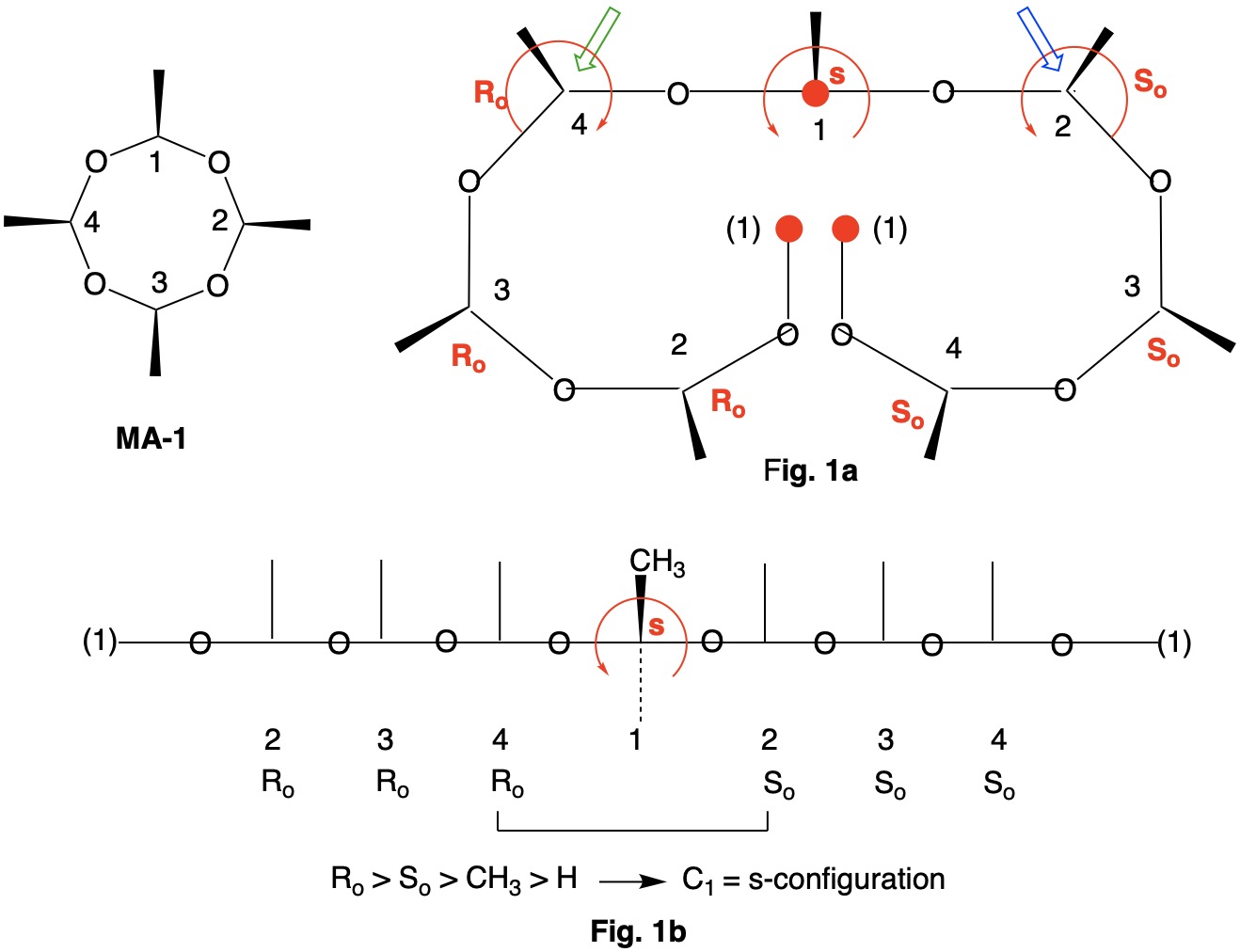
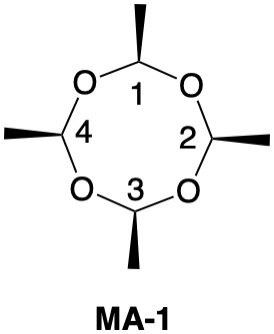
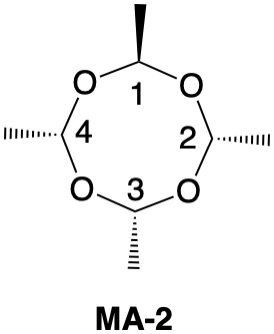
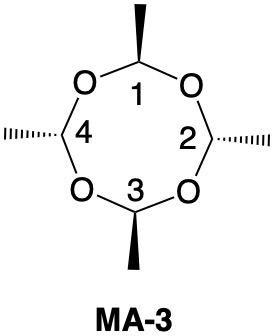
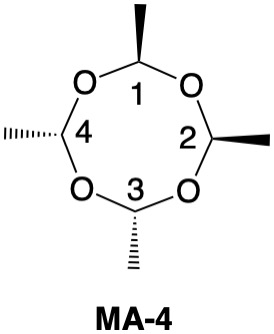
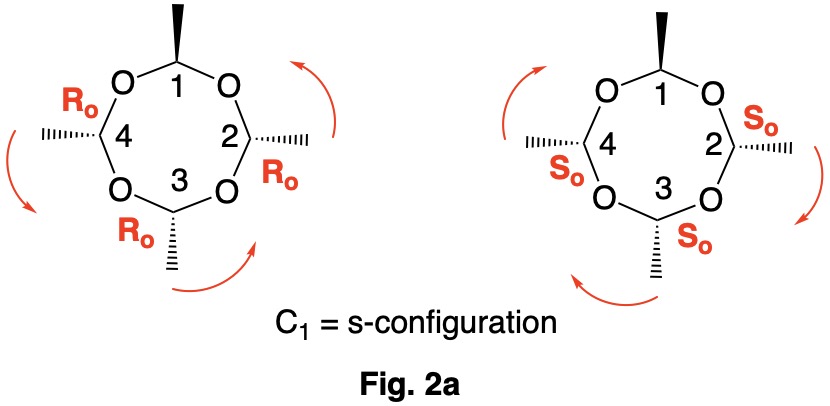
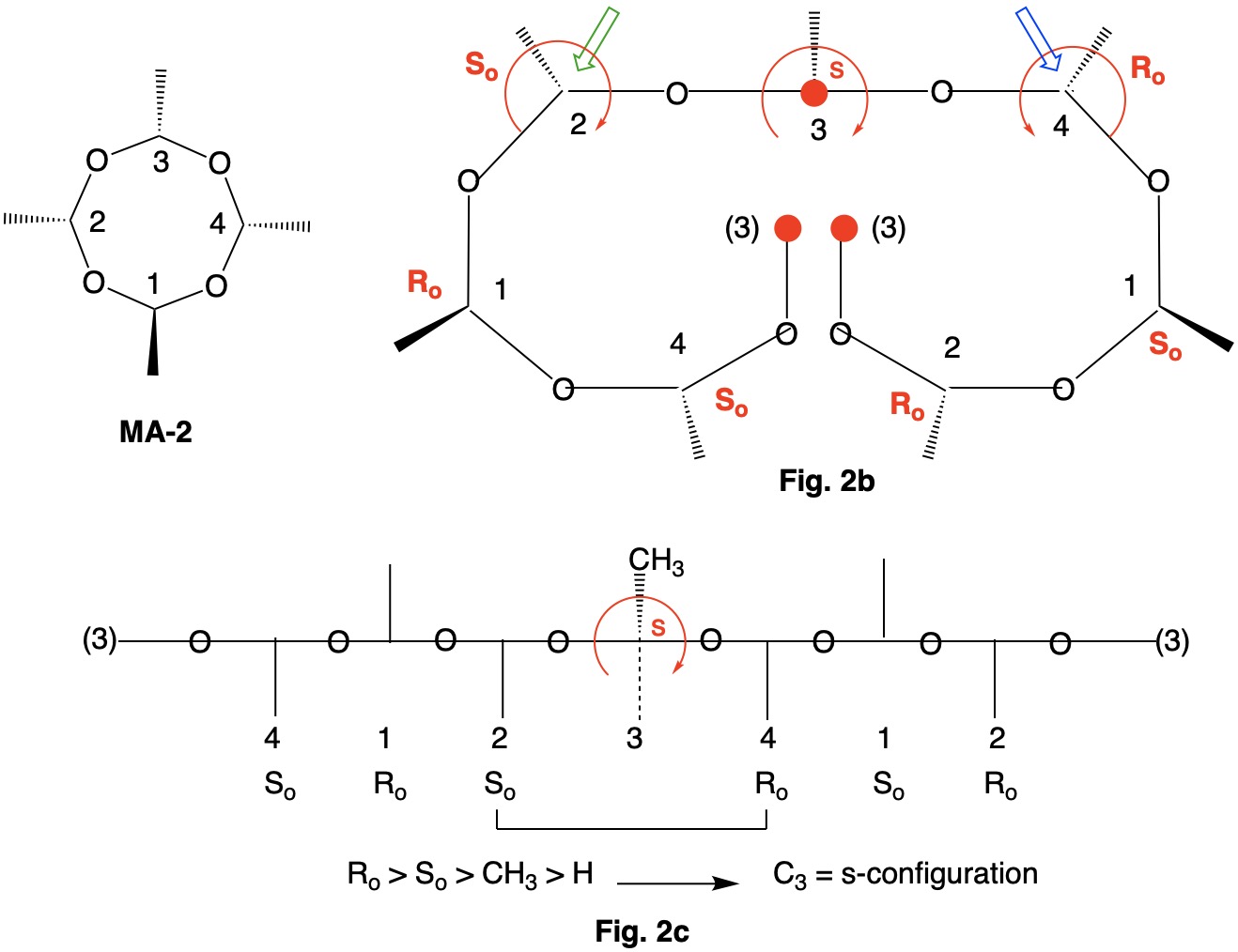
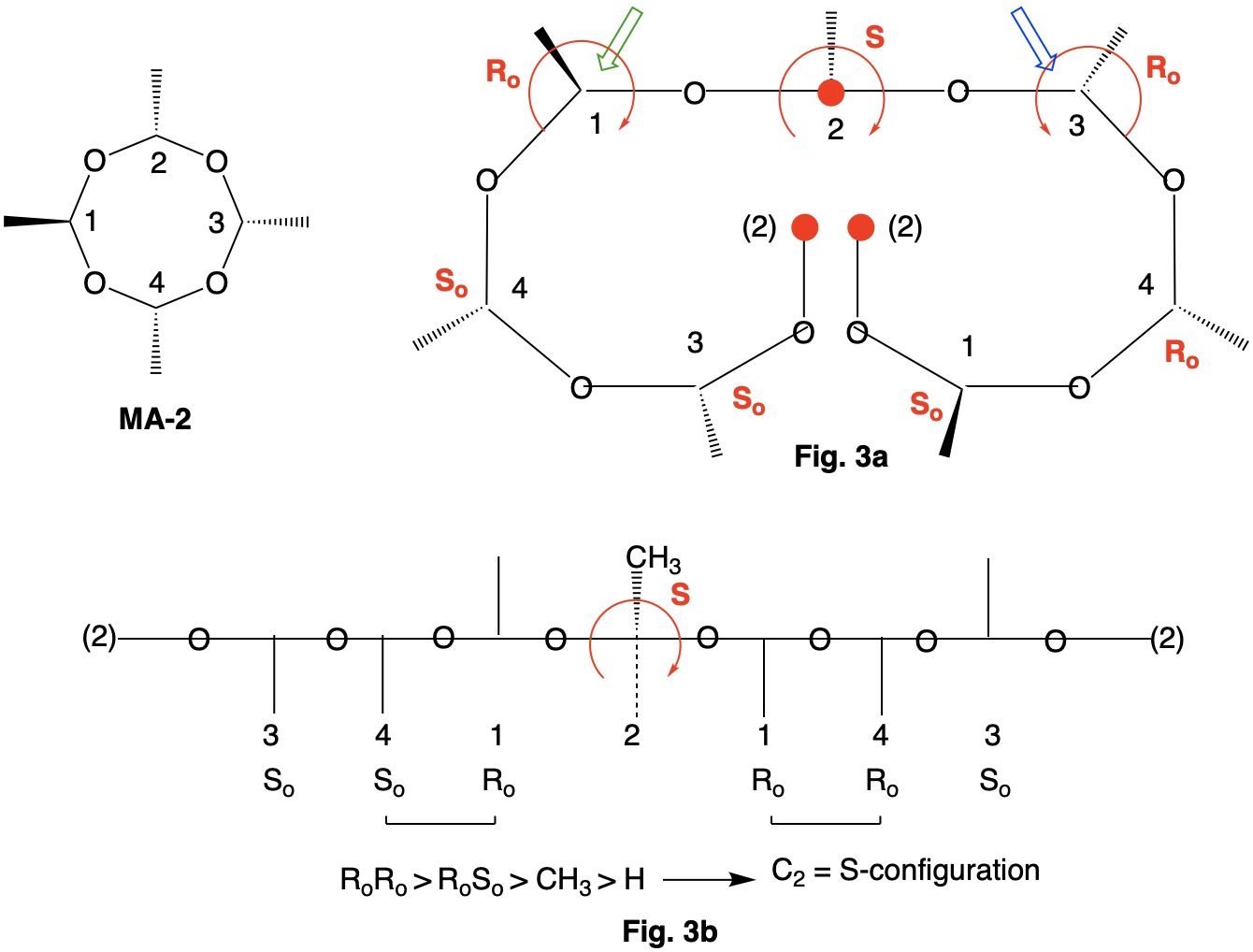
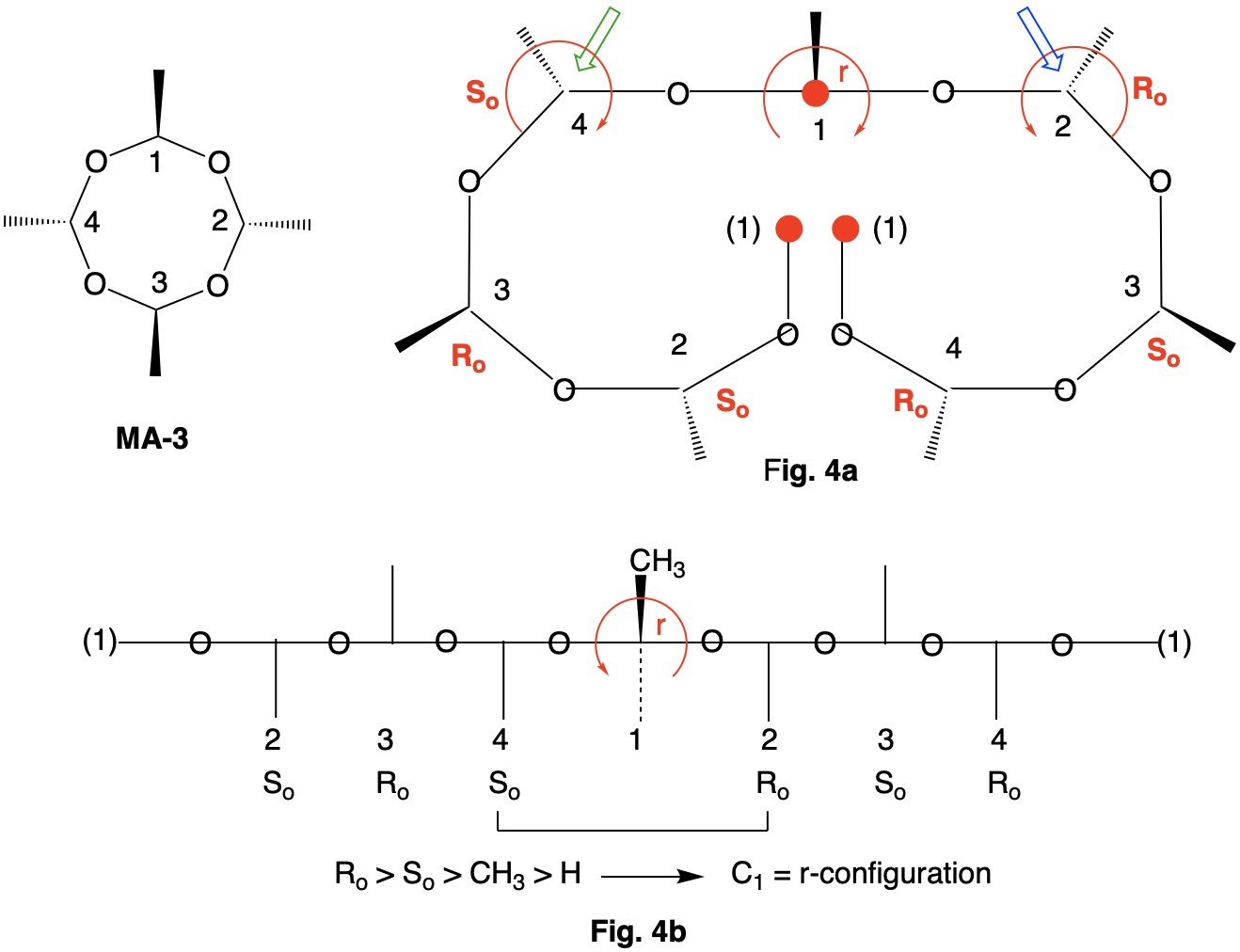
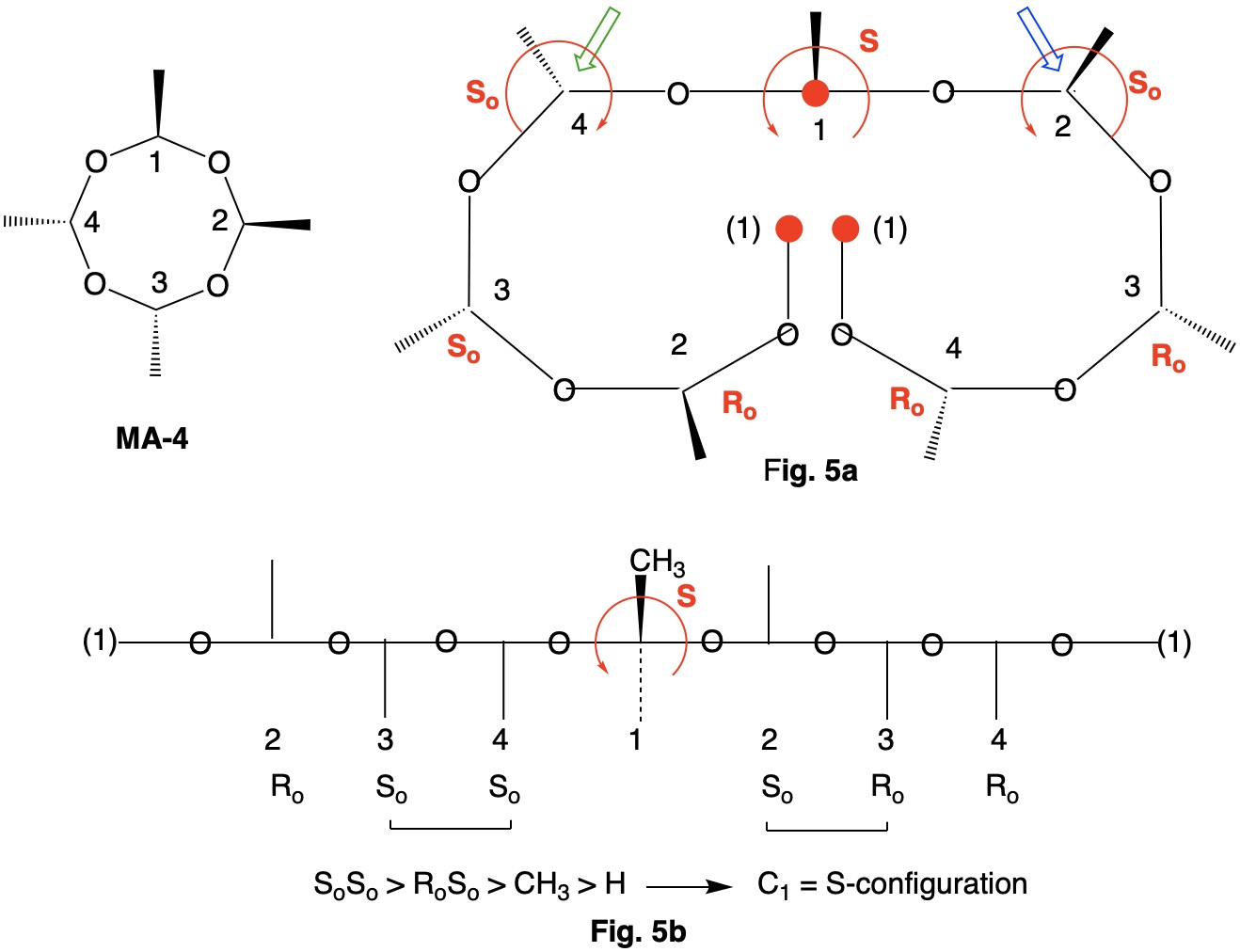
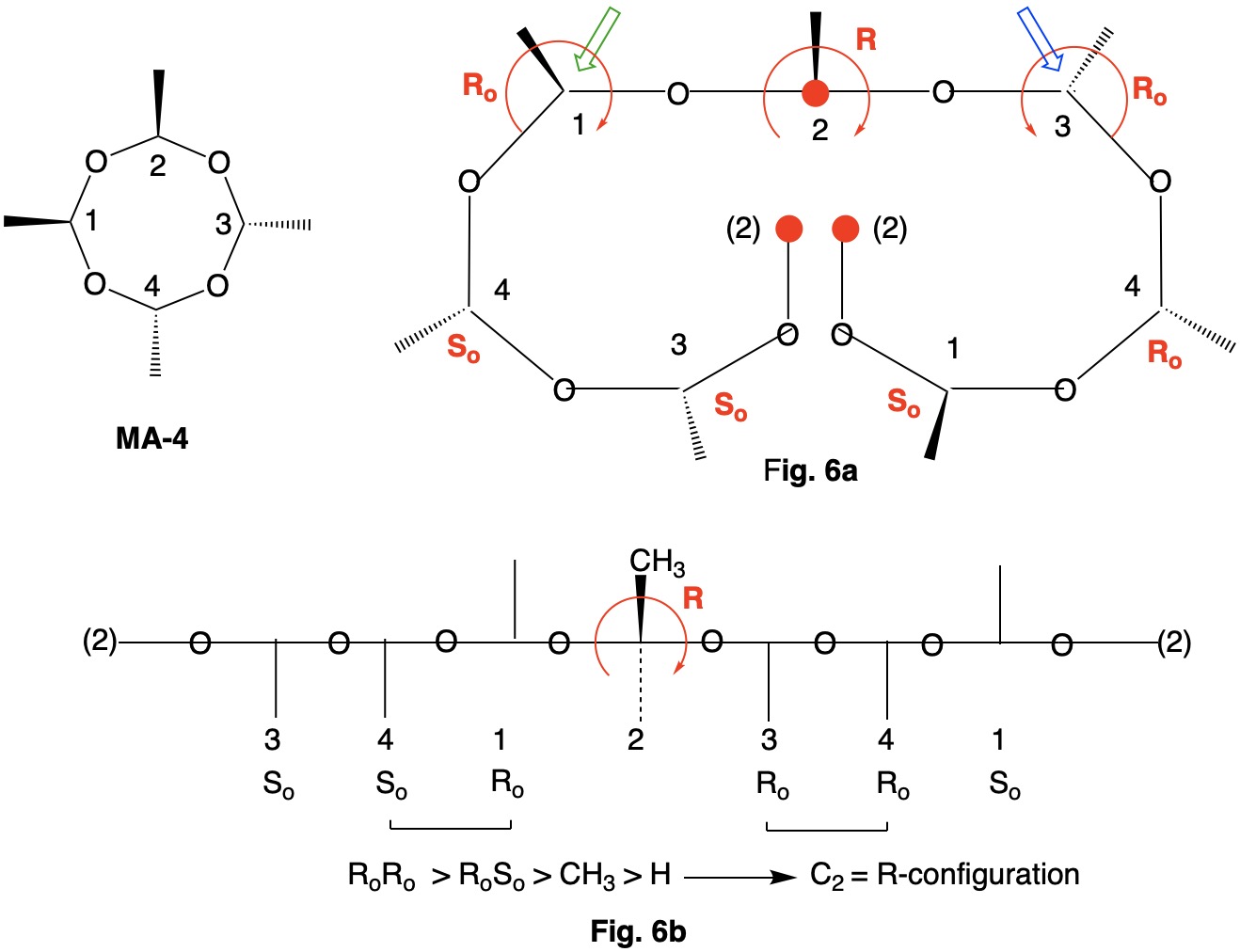
Metaldehyde MA-1: Metaldehyde (MA; 2,4,6,8-tetramethyl-1,3,5,7-tetraoxocane) is the tetrameric acetal of acetaldehyde. Four stereoisomers of MA are possible: MA-1, MA-2, MA-3 and MA-4. MA-1, which has the four methyl groups on one side of the ring, has planes of symmetry orthogonal to the tetraoxocane ring passing through C1-C3 and C2-C4. The two planes of symmetry through alternate oxygens is irrelevant. Carbons C1-4 are all stereogenic but achirotopic. Therefore, the CIP designation will be r/s and not R/S.
Careful examination of Fig. 1a shows that the left hand and right hand chains are mirror images of one another. Therefore, C2-4 of the left hand chain must be of the Ro configuration. You can test this claim at C4 by the green arrow. Now the configuration at C1 can be determined. Because the left hand chain has all Ro and the right hand chain has all So, one simply follows CIP Rule 5, Ro > So > CH3 > H. C1 has the s-configuration. Fig. 1b is another digraph representation. The horizontal line contains the ring atoms and the methyl groups are above this line. As noted early on in this discussion, all four ring carbons have the same s-configuration. Select the R/S;r/s button in the JSmol structure below for MA-1. |
|---|