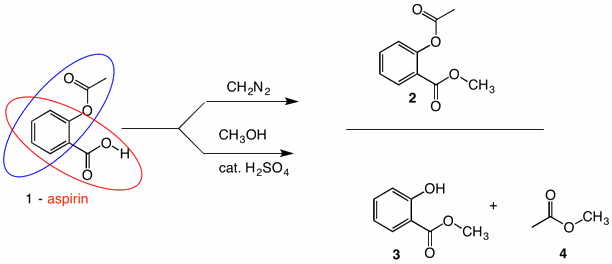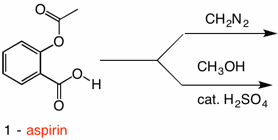
Solution: Fischer esterification of aspirin does not give its methyl ester 2 but rather methyl salicylate 3 (oil of wintergreen) and methyl acetate 4. Aspirin is both an aromatic carboxylic acid (red oval) and a phenyl ester of acetic acid (blue oval). While esterification will convert the carboxylic acid group to a methyl ester, transesterification (exchange of one alcohol portion of an ester for another alcohol) to afford methyl acetate 4 and methyl salicylate 3. The mechanism of transesterification is the same as acid hydrolysis of an ester (see ester #1). Now all is not lost. Methyl acetate is quite volatile and easily separable from methyl salicylate, which when treated with acetic anhydride, will form ester 2 upon acetylation. This procedure is ideal for larger scale preparations. But imagine that you only have 25 mg of aspirin and on top of that imagine that the acetyl group is isotopically labeled. You want minimal workup and no loss of the valuable acetyl moiety. Time for diazomethane.