Alkene 5:
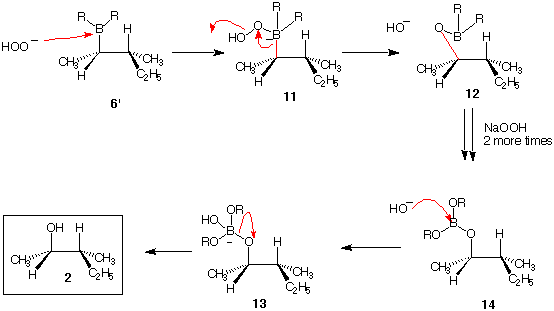
Let's assume that the hydroboration has proceeded with three moles of alkene 1 to produce 6' as opposed to 6. Hydrogen peroxide (H2O2) in aqueous NaOH forms NaOOH. When this anion adds to the Lewis acid borane, complex 11 is formed. Note that the atoms and charges are balanced. Na+ is ignored because it is not critical to understanding the reaction. The sp3 hybridized carbon orbital that is bonded to boron (red bond) migrates with its electrons to the electron deficient adjacent oxygen with the hydroxide ion acting as a leaving group to form 12. The red bond in 12 is now attached to oxygen without altering the stereochemistry at the carbon atom! The relative stereochemistry at the carbon in 11 bearing the boron atom is the same as the stereochemistry of the same carbon in 12 bearing the oxygen. [The astute student will notice that the R,S priorities have changed.] This oxidation is repeated with peroxide anion for each of the B-R bonds to give the borate ester 14. The difference between 6' and 14 is R-B has become R-O-B. When hydroxide adds to boron in 14, the alkoxides are liberated and protonated by water to form alcohol 2. The by-product is the trisodium salt of boric acid. Return.
