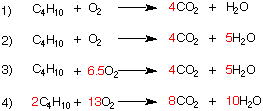
1) To balance this reaction, the number of carbons in CO2 must be the same as the number of carbons in C4H10 (n-butane).
2) The number of hydrogens in H2O must be the same as the number of hydrogens in C4H10 (n-butane).
3) The number of oxygens in CO2 and H20 (13) must be the same as the number of oxygen atoms in the moles of oxygen.
4) Because one cannot have a fractional number of moles in a balanced equation, equation 3 is multiplied by 2.
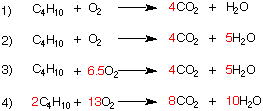
The heat (ΔHo) liberated in any chemical reaction is equal to the sum of the heats of formation of the products minus the sum of the heats of formation of the reactants. [ΔHo(rxn) = ΔHo (products) - ΔHo(reactants)] This is Hess's Law.
Using the heats of formation of the products and reactants of this chemical reaction (combustion), calculate the heat of combustion of n-butane at 25 oC. Assume that water is formed as gas. Check here to compare your answer.