Chem 220 - Organic Chemistry
Problem Set 3
Chapter 4, Chemical Reactions
Due: Monday, February 11, 2013
Solution Set
|
Dualism vs. Substitution
Theory
The prevailing theory of organic
structure in the early 19th century was Dualism or the
Electrochemical Theory, principally championed by Berzelius.
Since inorganic sodium chloride could be considered as
Na+Cl-, then an alkyl halide such as
RCl could be thought of as R+Cl-. The
R group or "radical" of its day, was thought to be
immutable, the carbons and hydrogens behaving as though they
were an element. Liebig (German) and Dumas (French), influential chemists of the
day, published a joint paper (1837), On the Present State of Organic Chemistry, extolling the
concept and claiming all that was left to do in organic
chemistry was to identify these immutable radicals (benzoyl,
ethyl, acetyl, etc.) As the story goes, a Parisian reception
at the Tuileries was to change all of this. [The Tuilerie Gardens was
one of the images in Mussorgsky's "Pictures at an an
Exhibition", composed in 1874 for piano and later
orchestrated by Ravel. The Tuileries selection is here.
For the history of this composition and background music, go here.]
The guests were discomforted by fumes from the burning
candles. Dumas was called in as a consultant. He found that
the waxes (fatty esters) had exchanged chlorine for
hydrogen, the culprit being the by-product hydrogen
chloride. [This story is likely apocryphal. It was
told by August
Hofmann at a eulogy for
Dumas (1884). If the event did occur, it is more likely that
the bleaching of candle wax involved addition of chlorine to
double bonds. Nonetheless, Dumas did investigate
substitution reactions.]
|

Jean-Baptiste-Andrè Dumas
(1800-1884)
|
The concept of exchanging electropositive
hydrogen for electronegative chlorine was anathema to
dualism. Liebig was not enamored with substitution. Why
shouldn't he be? After all Liebig and Wöhler had done precisely this in 1832 during their work on the
benzoyl radical (C7H5O). They had converted benzaldehyde
[(C7H5O)H] into benzoyl
chloride [(C7H5O)Cl] by the
action of chlorine. So disenchanted was Liebig with the
controversies regarding theory in organic chemistry, by 1840
he turned his attention to the practical applications of
agricultural chemistry. Thus arose Liebig's beef extract.
Dumas's student, Laurent, not one to
shirk from controversy, was bold enough to call the process
substitution rather than exchange. Thus, Substitution
Theory. Moreover, Dumas (1838) was able to substitute three
of the four hydrogens of acetic acid for chlorine to form trichloroacetic
acid, which had properties to
acetic acid. The recognition of these similar properties led
to early Type Theory. In 1842, Melsen, a student of Dumas,
reversed Dumas's experiment by reducing trichloroacetic acid
to acetic acid by the action of zinc metal. The promulgation
of Substitution Theory gave the wry
wit of Wöhler,
a.k.a., S. C. H. Windler, an opportunity to shine. At
the beginning of the 20th century free radicals were
detected and named free radicals to distinguish them from
the older radicals of Radical Theory of the early 19th
century. During the 19th century chemists tried to isolate
the older radicals to no avail. When Kolbe and
Frankland thought they had isolated methyl, they actually
had made the dimer of methyl, ethane. The very process of
substituting chlorine for hydrogen is a free radical
reaction. |
Study the Alkane Module in Organic
Reactions Go Online (ORGO).
|
Alexander Borodin (1833-1887), the
accomplished Russian composer, did not quit his day job. He
was a Professor of Organic Chemistry in St. Petersburg
although he is more famous as a composer.
He unknowingly discovered a method for the decarboxylation
of carboxylic acids (via their silver salts) in the presence
of bromine to afford an alkyl bromide with the liberation of
CO2. The reaction proceeds through a free radical
chain mechanism. Borodin did not isolate the alkyl bromide.
That accomplishment rested with Herr und Frau Hunsdiecker
(1942), after whom the reaction is eponomously named. |

CH3CH2CO2Ag
+ Br2 -----> CH3CH2Br +
CO2 + AgBr
|
1. Determine the percent of each of the constitutional monochloro
isomers expected from the chlorination of 3-methylpentane. Show
work.
|
C1 and C5 are equivalent primary carbons. Substitution of any one of these 6 hydrogens with chlorine will lead to 1-chloro-3-methylpentane. C6 is primary but different from C1 and C5. Why? There are four equivalent secondary hydrogens (C2 , C4) and one tertiary hydrogen at C3.
|

|
|
Carbon
Atom
|
# of
Hydrogens
|
Relative
Reactivity
|
Product
|
Fraction x
100
|
C1(5) primary
(1-chloro-3-methylpentane) |
6 |
1 |
6 |
18.5% |
C6 primary
(1-chloro-2-ethylbutane) |
3 |
1 |
3 |
9.2% |
C2(4) secondary
(2-chloro-3-methylpentane)
|
4
|
4.5
|
18
|
55.5%
|
|
C3
tertiary (3-chloro-3-methypentane)
|
1
|
5.5
|
5.5
|
16.9%
|
| Sum of Product column |
|
|
32.5 |
100% |
2. Show the initiation and propagation steps for
the free radical chlorination of cycloheptane.
See 2b.
a) Using Bond Dissociation Energies
(BDEs),
calculate the ΔHo for each relevant step and for the
overall reaction. Show work.
The initiation step is not
included in the heat of the reaction. It is catalytic. It gets
things going. In the 1st propagation step a secondary C-H bond is
broken (+95 kcal/mol) while H-Cl is formed (-103 kcal/mol). The
difference is -8 kcal/mol. The second propagation step is treated the
same way. Summing the two reactions gives -30 kcal/mol for the overall
reaction.
b) Calculate the heat of formation (ΔHfo)
of chlorocycloheptane. Use Heat
of Formation Tables. Show work.
|
Using heats of
formation one can get a value for cycloheptane, chlorine and
HCl. The overall heat of the reaction was determined
previously. Thus, the heat of formation of chlorocycloctane
may be calculated.
ΔHorxn = ΔHoproducts
- ΔHoreactants
-30 = (x -22.1) - (-28.2 + 0)
-30 = x + 6.1
x = -36.1 kcal/mol for the heat of formation of
chlorocycloheptane..
|

|
3. When cis-cyclooctene undergoes radical chain
bromination, only one monobromination product is isolated.
a) Draw the structures of the possible
monobromination products. Using BDEs,
explain which one is formed.
The weakest C-H bond is the
one that is allylic. It overlaps with the π-bond. Only
the circled bromide will form.
b) Write a radical chain mechanism for this
reaction.
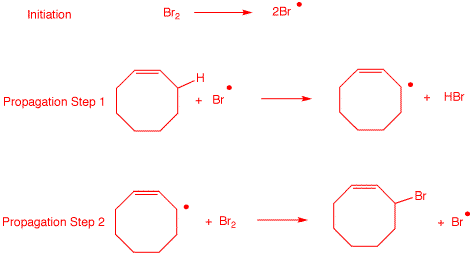
|
For this reaction to be successful,
bromine must be present in low concentration.
N-Bromosuccinimide (1, NBS) is
often used for this purpose (Wohl-Ziegler
reaction). Traces of HBr cause the formation of succinimide
2 and bromine as shown.
|

|
c) Carbon tetrachloride is the classic solvent for this reaction.
Benzene may also be used as a solvent but not toluene
(methylbenzene). Explain why carbon tetrachloride and benzene are
acceptable solvents but not toluene.
CCl4 has no CH
bonds and the CH bonds in benzene, like the vinylic CH bonds of an
alkene, have a BDE = 108 kcal/mol. The methyl hydrogens of toluene
are benzylic CH bonds: BDE = 85 kcal/mol. They are too reactive. The
benzyl radical is resonance stabilized.
|
d) The NBS free radical allylic
bromination of 1-methyl-1-cyclohexene (1) is not a
good preparative reaction. Although the five allylic
bromides 2-6 are the possible products, only bromides
3 and 6 are formed. Still, this is a difficult
separation.
i) How many unique allylic sites are there in alkene
1? Show them.
There are three: A
= methyl; B = methylene; C =
methylene.
ii) Which bromides are formed from each of the sites in part
i)? The bromides
are formed in the second propagation step. The resonance
structures of the radicals are shown along with the bromides
that form.
iii) What does the fact that only
3 and 6 are formed tell you about the allylic
methylene group vs. the allylic methyl group? Karl Ziegler recognized
early on that allylic methyl groups are less reactive than
allylic methylene groups.
Generally, the
bromide with the more substituted double bond is preferred:
Excluding path A,
C prefers 3 and
not 5.
iv) How could you have
anticipated this relative reactivity?
Non-allylic
methylenes are also more reactive than non-allylic methyl
groups.
|

|
4. After reading "Hess's
Law of Constant Heat Summation" and/or
"Heats of Combustion, Heats of Formation, and Bond Dissociation
Energies (Powerpoint)" in the Thermochemistry
Module, answer the following :
a) Draw a Standard State diagram that illustrates
how to calculate the ΔHfo of an n-propyl
radical. Make use of the BDEs
and Heats
of Formation Tables.

b) How does your answer in a) compare with the
value in the Heats of Formation table?
You will notice that an n-propyl radical is not listed but an ethyl radical has a heat of formation of +26 kcal/mol. Since an n-propyl radical has one more -CH2- than an ethyl radical,the heat of formation of the n-propyl radical should be -5 kcal/mol less than an ethyl radical or ΔHfo = +21 kcal/mol. On the money!
c) Using the value obtained in a), illustrate and
calculate the C3-C4 bond energy in
n-hexane. Here we work the problem in
reverse. In a) we see that:
ΔHfo(R-R)
+ ΔHo(R-R) = 2 x ΔHfo(R.),
therefore
Δ
Ho(R-R)
= [2 x
ΔHfo(R.)]
- ΔHfo(R-R)
ΔHfo(R-R)
= [2 x
21]
- (-40) = 82 kcal/mole






