|
Some of the problems in the alcohol module in ORGO will serve to review the chemistry of this chapter. As you master the chemistry of alcohols, you should try the Web of Reactions. You will not be able to do all of them. 1. How many grams of KMnO4 in aqueous KOH are required to oxidize 30 grams of 1-pentanol to pentanoic acid (valeric acid)? [Note: MnO2 is the reduction product of permanganate. This is a redox reaction from Gen. Chem. Go here for help. Derive the balanced equation and show your work.]. In alkaline medium the element balanced reduction of manganese is MnO4- + 2H2O ----> MnO2 + 4OH- (+3 electrons) The balanced elemental oxidation of 1-pentanol is CH3(CH2)3CH2OH + 4OH- ----> CH3(CH2)3CO2H + 3H2O (-4 electrons) Multiplying the reduction by 4 and the oxidation by 3 gives 4MnO4- + 8H2O ----> 4MnO2 +16OH- (+12 electrons) 3CH3(CH2)3CH2OH + 12OH- ----> 3CH3(CH2)3CO2H + 9H2O (-12 electrons) adding these equations gives 4MnO4- + 8H2O + 3CH3(CH2)3CH2OH + 12OH- ---> 3CH3(CH2)3CO2H + 9H2O + 4MnO2 +16OH- Simplifying gives 4MnO4- + 3CH3(CH2)3CH2OH ---> 3CH3(CH2)3CO2H + H2O + 4MnO2 + 4OH- Four moles of KMnO4 accommodates 3 moles of alcohol. 1-pentanol: MW = 88.15; 30 g = 0.34 moles 0.34 moles x 4/3 = 0.45 moles of KMnO4 KMnO4: MW = 158.03 (158.03 g/mol) x (0.45 moles) = 71.11 g of KMnO4 |
Paul Sabatier (1854-1942) Co-Nobel Prize in Chemistry (1912) Catalytic Hydrogenation |
Victor Grignard (1871-1935) Co-Nobel
Prize in Chemistry (1912) Organomagnesium Compounds |
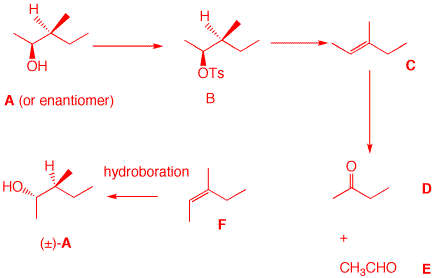
2. Optically active compound A (C6H14O) reacts with tosyl chloride/pyridine to form B. Treatment of B with KOH solution affords optically inactive compound C. Ozonolysis and dimethyl sulfide reduction of C provides D (C4H8O) and E. Compound D is inert toward aqueous chromic acid. Compound C is more stable than its stereoisomer F. What are the structures A-F? What is not known about A? Show your reasoning. How would you efficiently prepare (±)-A from F? Compound A has no degrees of unsaturation.It is an alcohol because it reacts with tosyl chloride to form tosylate B. C is an alkene because it undergoes ozonolysis. B ---> C is an E2 elimination. D is either an aldehyde or ketone. Because D is inert to aqueous chromic acid, D is a ketone. Only 2-butanone is possible for D. Compound E has two carbons. Only acetaldehyde is possible. A is either (E)- or (Z)-3-methyl-2-pentene. The (E)-isomer has two methyls syn to one another whereas the (Z)-isomer has a methyl and an ethyl syn. C is the (E)-isomer; F is the (Z)-isomer. See the Heats of Formation table. One doesn't know which enantiomer of A is present! Hydroboration of F adds water in a syn anti-Markovnikov sense to give racemic A. In the elimination of B, the elimination was anti.
|
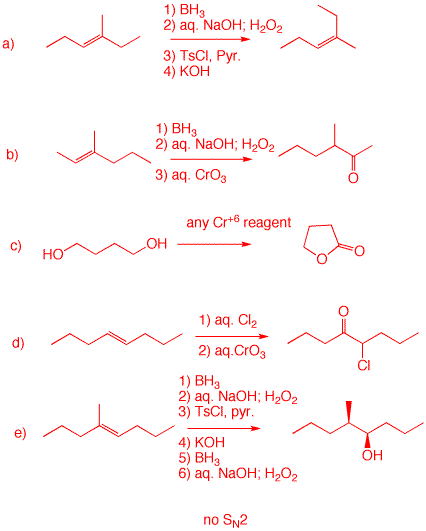
3. Provide reagents for the completion of each of these reactions. No non-selective reactions. Justify your answers. a) Add water anti-Markovnikov syn; remove water (as TsOH) by anti E2 elimination. Invert sterochemistry of the alkene. b) Make an alcohol via hydroboration. Oxidize with any Cr+6 reagent. c) Oxidation of one primary alcohol will form an aldehyde, which will intramolecularly "hydrate" with the alcohol group forming a hemiacetal. This species will oxidize with the reagent to the lactone (intramolecular ester. d) The alkene is symmetrical forming a single chlorohydrin. Chromic acid oxidation of the secondary alcohol to the chloroketone. e) The first 3 steps effectively add HOTs syn to the double bond in an anti-Markovnikov sense. KOH effects E2 anti elimination, converting the original alkene to the (Z)-isomer. (see part a.) Syn hydroboration gives the alcohol. (Remember 4b on exam 2. Click here.)
|
|
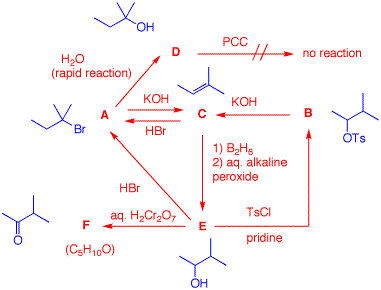
4. A laboratory bottle has the label "C5H11Br". A student decides to run some reactions on the contents of the bottle to determine the structures of the compound A. What are the structures A-F? Show your reasoning. [Hint: Draw all of the structures of C5H11Br. Eliminate non-contenders? Only the major product in the formation of C should be considered.] The major clue is the rapid reaction of the bromide A with water. There is only one C5 tertiary bromide.. This is an SN1 reaction to give tertiary alcohol D. A can give Zaitsev elimination to form C and add HBr in the Markovnikov sense to regenerate A. Hydroboration of C gives alcohol E. Oxidation gives a ketone F (not an aldehyde; no over oxidation) while treatment of E with tosyl chloride affords tosylate B. Zaitsev elimination of B gives C.
|
|
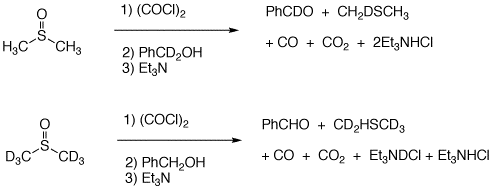
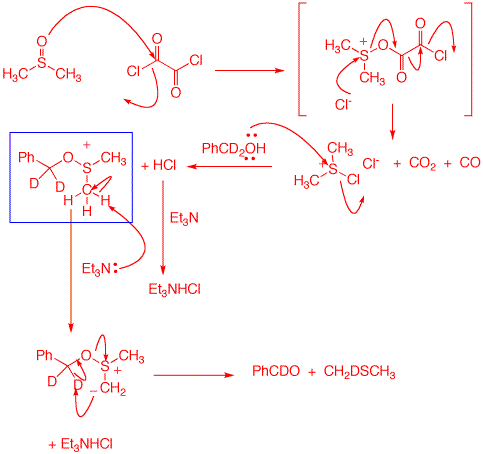
5. Consider problem 10.56 on pg. 480. Studies on the mechanism of Swern oxidation provide the results of two experiments. a) How do these results compare with the mechanism shown in the Study Guide? The mechanism in the Study Guide is not in accord with these results. b) The Swern oxidation does not mix everything together as you may conclude from the text. There are three steps involved. Using the first reaction, show the products formed in each step with a mechanism for each. [Hint: Step 1 produces CO and CO2.] |
 |
|---|
The mechanism on the right explains the result as would experiment #2. The steps up to the sulfoxonium salt (blue box) are the same in both mechanisms. The data argues for Et3N to deprotonate the salt to form an ylid. This zwitterion acts as an internal base to generate the deuterobenzaldehyde and monodeuteriodimethyl sulfide. Using the Study Guide mechanism, the Et3N acts as an external base forming the same deuterated aldehyde but the lost deuterium would be in Et3NDCl and afford all protio dimethyl sulfide. The Guide has the wrong mechanism. Even Wikipedia has the correct mechanism for the Swern oxidation. |
 |
|---|