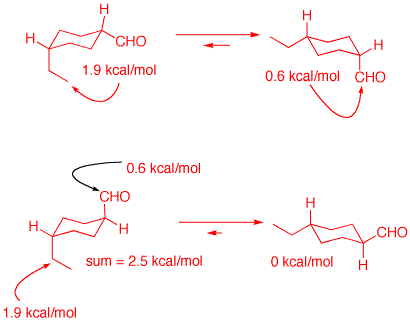
The Baeyer Laboratory, Munich, 1893
(This photograph is in the hallway across from 110 SCL)
Adolf von Baeyer (1835-1917); Nobel Prize 1905. (center, seated with derby), who was a student of Kekulé, succeeded Liebig at Munich. In the photograph (second row; third from right) is Henry Lord Wheeler (1867-1914); Yale Faculty 1896-1911. As was the custom in the 19th century, many Americans, such as Wheeler, did advanced study in chemistry in Europe. Karl is the laboratory assistant. (The only person wearing an apron and no tie; upper left.)
In 1885, as an addendum to a paper on acetylenic compounds, Baeyer proposed that cyclopentane was the least strained of the cycloalkanes
.
While he accepted the idea that the carbon atoms in
cycloalkanes were tetrahedral, he treated the cycloalkanes
as though they were flat. He argued that there is only one
cyclohexane carboxylic acid, not two (axial and equatorial)
as was predicted by a chair cyclohexane.
Equatorial is frequently misspelled.

Sir Derek H. R. Barton (1918-1998)
1969 Nobel Prize with Odd Hassel for their work on conformational analysis
For a video of Barton talking about conformational analysis, click here.
Cyclohexane in the chair conformation