Chem220 - Organic Chemistry
Problem Set 4
Solution Set
Chapter 5: Alkenes: Addition Reactions
Due: Monday, October 10, 2011
In 1840, Christian
Friedrich Schönbein (1799-1868) discovered ozone (Gr.; odorant), the sharp
odor produced by electrical discharges. Seven years later
(1847) he observed that ozone oxidizes organic compounds
but not to their ultimate products of oxidation, carbon
dioxide and water. [Two years prior, he had spilled
nitric and sulfuric acid on his Frau's apron in her
kitchen. The apron, made of cotton, combusted and thus
was discovered gun cotton, nitrocellulose. Schönbein
also observed that hydrogen peroxide (Threnard; 1818) is
oxidized to oxygen gas in the presence of hemoglobin.
] In the period 1903-1916, Carl
Dietrich Harries (1866-1923),
an assistant to both Hofmann (of the eponymous
elimination and rearrangement) and Fischer (of projection
and carbohydrate fame) at Berlin, published some 80
papers on the reactions of ozone with organic compounds.
His interest was stimulated by the reaction of ozone with
rubber, a process that causes rubber to become hard and
brittle. These studies led to the analytical and
synthetic uses of ozone. From 1904-1916 he was a
professor at Kiel. Disenchanted with academic life, he
became Director of Research for Siemens and Halske, the
German company co-founded by the electrical pioneer,
Werner von Siemens, his father-in-law. Not surprisingly,
Siemens went into the business of producing ozone
generators. The studies of Rudolf Criegee (1902-1975; Karlsruhe)
produced a unified mechanism for the process
of ozonolysis.
M.
Rubin, Bull. Hist. Chem., 2001, 26,
40.
M. Rubin, Helv. Chem. Acta, 2003, 86, 930.
Reading Assignments:
a)The alkane and alkene modules
in ORGO will help with this Problem Set.
[Note that some of the problems in the alkene module deal with stereochemical issues. We will get there.]
b) Ozonolysis module.
|

Rudolf Criegee
(1904 - 1975) |
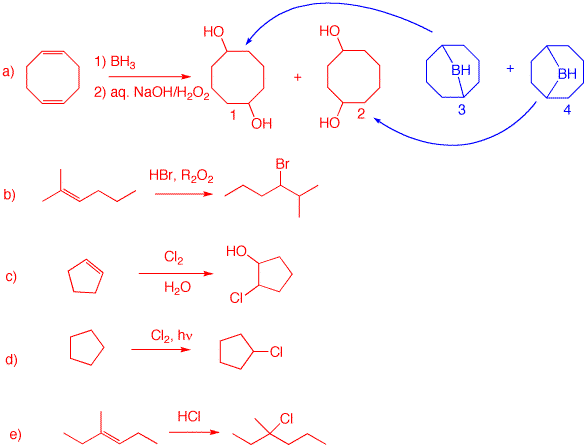
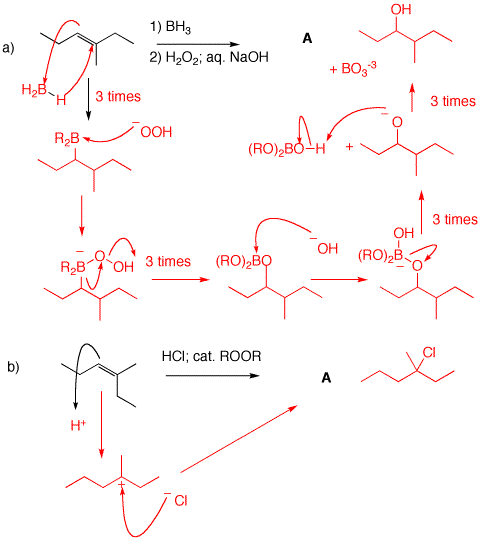
1) Predict the major product in each of the following reactions. Use the curved arrow formalism to illustrate how each intermediate is formed.
a) Boron is less electronegative than hydrogen. Boron adds to the less substituted end of the double bond while hydrogen (hydride) adds to the more substituted end. [Note: Highly substituted alkenes may not react 3:1 with borane. Peroxide anion is formed with NaOH. The anion adds to boron followed by carbon migration to oxygen losing hydroxide. Hydroxide adds to boron liberating the alkoxide of the alcohol. Protonation gives the alcohol A and sodium borate. The overall reaction is an anti-Markovnikov addition of water to the double bond.
b) Peroxide has no effect on HCl addition like it does with HBr. The addition of HCl is in the Markovnikov sense.
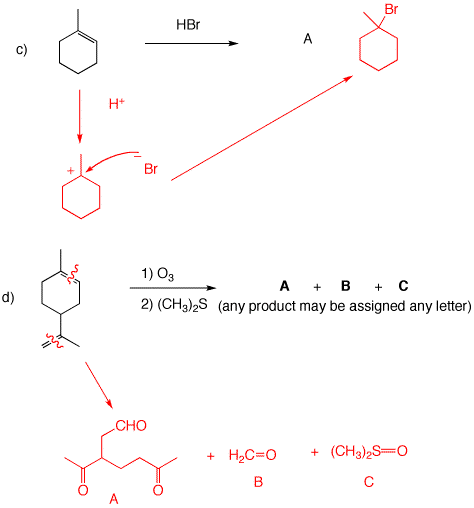
c) The mechanism is the same as in b), except that HBr is used instead of HCl. Normal Markovnikov addition; no peroxide effect.
d) Only the products are listed with no mechanisms. For details you should have read the reading assignment on ozonolysis. The compound is the monoterpene, limonene (C10H16). It is the fragrance in lemon grass. The trisubstituted double bond, which is contained in a ring, affords a ketone and an aldehyde attached by a carbon chain. The 1,1-disubstituted double bond yields formaldehyde (C1) and a ketone which along with the previously mentioned ketone and aldehyde gives the diketoaldehyde A as a C9 compound. C is dimethyl sulfoxide formed by the reduction of the ozonide with dimethyl sulfide. It is not possible to determine the structure of limonene from A and B without additional information. There are three possible structures that could be consideredfor limonene by reconstituting tthe ozonolysis products. Can you draw them?
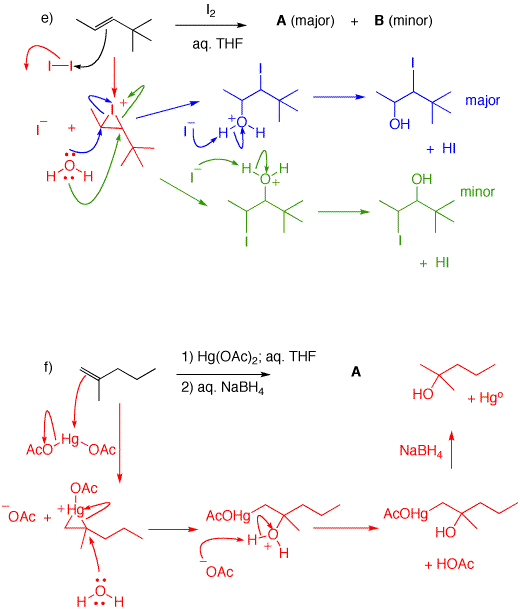
e) THF (tetrahydrofuran) is a cyclic ether misible with water in all proportions. It serves to dissolve the alkene and the iodine. Water is the nucleophile. An iodonium ion is formed. Water has two possible secondary sites to attack the iodonium salt. Presumably, the blue arrows indicate the less hindered site (methyl group) for attack while the green arrows display the more hindered route (adjacent to the tert.-butyl group). A = 3-Iodo-4,4-dimethyl-2-pentanol; B = 2-Iodo-4,4-dimethyl-3-pentanol.
f) A cyclic mercurinium ion is formed. Water attacks nucleophilically at the more hindered site to give an alkyl mercurial alcohol. The transition state is one where there is some positive charge developed on carbon. Clearly, the more substituted site is better at stabilizing a positive charge. Reduction of the C-Hg bond is a complex free radical reaction.
|
|
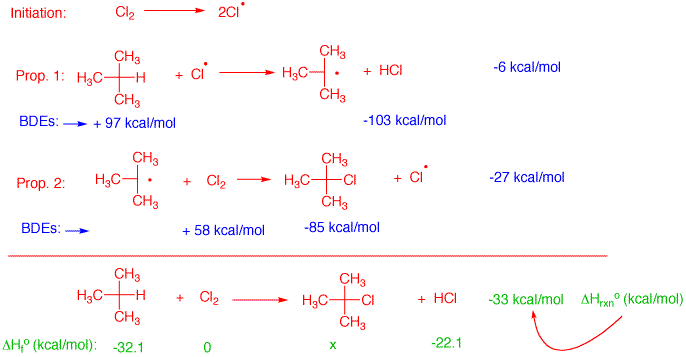
2) Study problem #1 in the Alkane Module in ORGO. Using bond dissociation energies, calculate the heat of each propagation step and the heat of the overall reaction. Now determine the heat of formation of 2-chloro-2-methylbutane using the heats of formation table. The reaction is initiated with light to generate some chlorine atoms. Consequently, this step does not contribute to the heat of the reaction. In the first propagation step a tertiary C-H bond is broken. Breaking bonds costt energy (endothermic), thus, a positive value. Since alltertiary C-H bonds have the same BDE, use the value for isobutane, 97 kcal/mol. Formation of HCl is exothermic, -103 kcal/mol. The heat of the first propagation step is exothermic by -6 kcal/mol, the rate limiting step. Similarly, the second propagation step liberates -27 kcal/mol. Thus, the overall reaction is exothermic by -33 kcal/mol. The heats of formation of both reactants are available along with that of the product HCl in the heats of formation tables. Since ΔHfo(rxn) = ΔHfo(prod) - ΔHfo(react),
ΔHfo(rxn) = -33 = [x + (-22.1)]- [(-32.1) + 0] = -43.0 kcal/mol
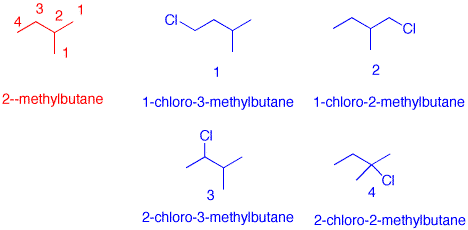
3) There are four possible constitutional isomers arising from the free radical monochlorination of 2-methylbutane. The relative reactivity of C-H bonds sp3 hybridized alkanes toward chlorine atoms is: primary, 1; secondary, 4.5; tertiary, 5.5.What are the structures of these monochloro compounds (C5H11Cl)? How much of each one is formed? Show work. 2-Methylbutane has 3 methyl groups, 2 of which are identical (2 x C-1). There are 6 C-1 hydrogens, 3 at the C-4 methyl group, 2 secondary hydrogens at C-3 and 1 tertiary hydrogen at C-2. Multiplication of the number of hydrogens times the reactivity gives a product for each group. The sum of the products is 23.5. The % is the fractional contribution of eac product. Notice that compound 3 is predicted to be the major product even though C-3 of 2-methylbutane has 2 hydrogens and C-1 has 6. The reactivity makes the difference.
2-methylbutane |
#H's |
Reactivity |
Product |
% |
| C - 1 (#2) |
6 |
1 |
6 |
25.5 |
| C - 2 (#4) |
1 |
5.5 |
5.5 |
23.4 |
| C - 3 (#3) |
2 |
4.5 |
9 |
38.3 |
| C - 4 (#1) |
3 |
1 |
3 |
12.8 |
| Sum |
|
|
23.5 |
100 |
|
 |
4) Hydroboration and oxidation of either (E)- or (Z)-3-hexene forms 3-hexanol efficiently. Why? Can the 2-hexenes be substituted for 3-hexene? Explain. Only one alcohol is formed in the hydroboration of either 3-hexene, namely 3-hexanol. On the other hand, the 2-hexenes would provide a mixture of 2-hexanol and 3-hexanol. Non-selective reactions are not desireable because separation is often difficult.
5) Provide reaction conditions for each of the following reactions. Provide commentary.
a) Hydroboration of of 1,5-cyclooctadiene gives boranes 3 and 4. The remaining B-H bond does not react. Treatment of the boranes with alkaline peroxide givesthe respective diols 1 and 2.
b) The product requires anti-Markovnikov addition of HBr. Use a peroxide with HBr.
c) Chlorine forms the intermediate chloronium ion wich is opened by water to give 2-chlorocyclopentanol.
d) Markovnikov addition of HCl to a trisubstituted double bond.