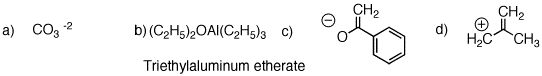Problem Set 1
Solution Set
Chapters 1 and 2, Structure, Bonding, Alkanes
Due: Monday, September 19, 2011
John Dalton (1766-1844) John Dalton's formulation of an Atomic
Theory in the first decade of the
19th century provided a theoretical basis for understanding
chemical behavior. In addition to defining the Law of
Multiple Proportions, he also formulated the Rule of
Greatest Simplicity, which held that water was a binary
compound, OH. (Note: Dalton did not use our modern symbols,
which came to us from Berzelius,
but rather
circles that were distinguishable
from one another.) Dalton established the combining masses
of H to O in water as ~1:6. This ratio was later refined to
1:8. Dalton postulated that in a molecules comprised of two
different atoms, the simplest one in the series would be
binary. While this rule applied to CO and CO2, it
did not apply to the pair, water and hydrogen peroxide.
Thus, water, according to Dalton, was OH. The Rule
of Greatest Simplicity, which was
at odds with Gay-Lussac's
Law of Combining Volumes of Gases that demonstrated the
volume of hydrogen produced upon electrolysis of water was
twice that of oxygen, was dismissed by Dalton as a faulty
result. Moreover, although there was agreement regarding the
combining masses of atoms in the first half of the
nineteenth century, there was
disagreement as to the unit mass
of the common atoms encountered in organic chemistry:
hydrogen (1), carbon
(2x6 or 1x12), oxygen (2x8 or
1x16). Since hydrogen was the lightest of the elements, it
was assigned a mass of one (Prout's
Hypothesis), a notion that is
unrelated to today's mass of hydrogen owing to the presence
of a single proton in the hydrogen nucleus. Berzelius's
proposal of a mass scale based upon O = 100 would have
worked as well. For a Brief History of Organic Chemistry
(PowerPoint), click
here.
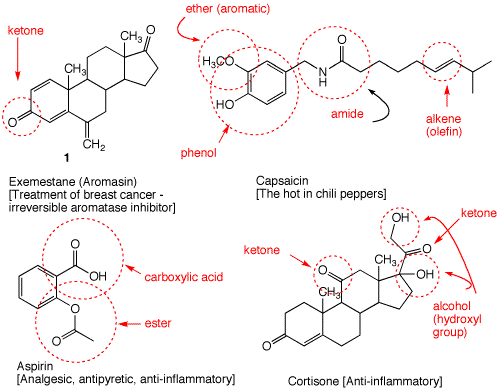
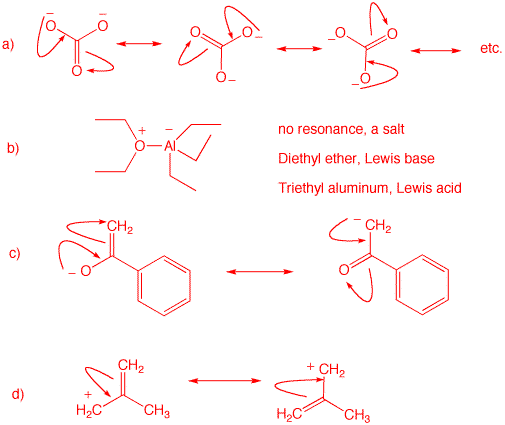
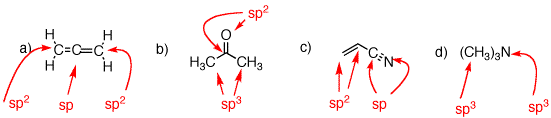
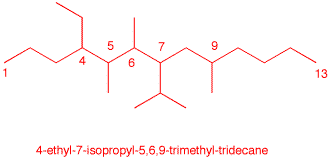
1. Identify the functional groups in the red circles. The front inside cover of your text will be of use. Complete this problem on a copy of this page and attach it to your homework.