,
Problem Set 6
Chapter 7, Structure and Synthesis of Alkenes
Due: Monday, October 18, 2010
Notice:
For those of you who are using the 6th edition of the
textbook, you can learn about olefin metathesis by
clicking
here. 1. Read Degree
(Elements) of Unsaturation. How
many degrees of unsaturation are present in
C16H13ClN2O? Draw two
structures, one cyclic, the other acyclic, that have the
number of degrees of unsaturation you determined and that is
necessarily in agreement with the formula. b) Calculate the heat of
hydrogenation of (E)-and
(Z)-3-methyl-2-pentene. Show work. How
to manipulate JSmol structures. 4. The major product B from free
radical monobromination of alkane A
(C6H14) readily reacts with water to
form C, C6H14O. Treatment of
bromide B with KOH in ethanol produces two, and only
two, compounds, D and E. Compound D
liberates 1.6 kcal/mol more heat upon hydrogenation to
A than does E. What are the structures
A-E? What is the heat of isomerization of D to
E? Illustrate and explain. [Hint: What are
the structures possible for A?] 5. In 1968 Wiberg
and Fenoglio determined the heat of combustion of
cyclopropene: -485.0 kcal/mol. Determine the heat of
hydrogenation of cyclopropene to cyclopropane. Explain and
illustrate with a diagram. 6. Two stereoisomers, A and
B, absorb one equivalent of hydrogen upon catalytic
hydrogenation to form cyclooctane. Compound A, which
is capable of resolution, liberates 34.5 kcal/mol of heat
while B liberates 24.3 kcal/mol of heat.
2. a) Determine the heat of formation of 3-methyl-1-pentene.
Use the heat
of formation tables to determine
typical heats of hydrogenation for monosubstituted alkenes
and to determine the heat of formation of the the reduction
product of 3-methyl-1-pentene. Show work.
c) Use a diagram to illustrate that the difference in the
heat of hydrogenation of the two geometrical isomers in 2b
is equal to the difference in their heats of formation.
Which isomer is more stable based upon the heats of
formation? Why?
d) There is only one monosubstituted alkene having the
carbon skeleton of the (E)-and (Z)- isomers in
2b. What is its structure? Assuming that ΔGo
= ΔHo, which of the three isomeric alkenes
would dominate in an equilibrium mixture? How much heat is
liberated in the isomerization of the monosubstituted alkene
to the (E)-isomer? Show work. Add the monosubstituted
alkene to your diagram in 2c and illustrate the heat of
isomerization.
Paul
Sabatier
1912 Co-Nobel Prize in Chemistry
Hydrogenation by Metal Catalysis
3. a) Using the standard values for the relative reactivity
of 1o, 2o and 3o C-H bonds
toward free radical chlorination, determine the relative
amount of the four possible monochlorides formed from the
chlorination of [2.2.1]-bicycloheptane.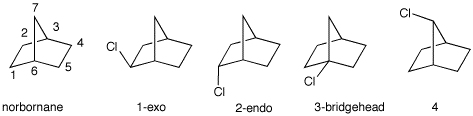
b) The experimental result is: 1, 72%; 2, 25%;
4, 3%; 3, 0%. Why is no bridgehead isomer formed even
though tertiary C-H bonds are highly reactive?
c) There are two types of secondary radicals formed. What is
their relative reactivity? [Hints: Measure the
C2-C1-C6 and the
C3-C7-C6 bond angles in
norborane on the right in which the radical is formed on the
central carbon. How do these values compare with
cyclopentane?
The chlorination of cyclobutane is slower than the
chlorination of cyclpentane.]
d) At what point in the radical chain are the endo and exo
isomers formed? Why does the exo isomer dominate? Explain
and illustrate.
e) Are the four monochlorides optically active, racemic, or
achiral?
a) What are the structures of A and B ?
b) What are the heats of formation of A and B
?
c) What is the difference in strain energy between A
and B ?
d) What is the difference in the
heat of combustion between A and
B?
e) Why is A capable of
resolution?