|
Notice: For those of you who are using the 6th edition of the textbook, you can learn about olefin metathesis by clicking here. 1. Read Degree
(Elements) of Unsaturation. How
many degrees of unsaturation are present in
C16H13ClN2O? Draw two
structures, one cyclic, the other acyclic, that have the
number of degrees of unsaturation you determined and that is
necessarily in agreement with the formula. Dropping O gives C16H13ClN2. Drop Cl, add one H: C16H14N2 Each N is worth CH; therefore, C18H16 The most saturated C18 hydrocarbon is C18H38. (38-16)/2 = 11 degrees of unsaturation. If you like formulas, let's derive one from what was done above. The number of hydrogens in the most saturated hydrocarbon must be 2C + 2 less the number hydrogens, halogens and nitrogen equivalents all divided by 2. Since the number of carbons in the most saturated hydrocarbon is increased when nitrogen is replaced by CH, one has D.U. = {[2(C+N)+2]-(H+X+N)}/2. This equation can be reduced to D.U. = C-((H+X-N)/2)+1. For the case at hand, D.U. = 16-(12/2)+1 = 11. Two structures are below. The cyclic structure happens to be valium (About its inventor, Leo Sternbach). 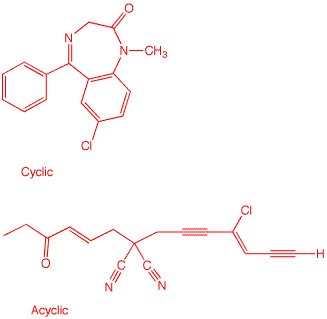
|
Paul Sabatier 1912 Co-Nobel Prize in Chemistry Hydrogenation by Metal Catalysis
|
|
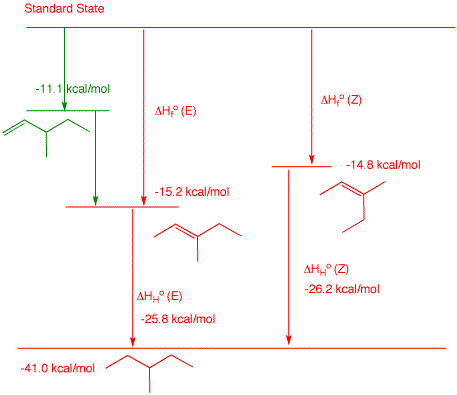
2. a) Determine the heat of formation of 3-methyl-1-pentene by using the heat of formation tables to determine typical heats of hydrogenation for monosubstituted alkenes. Show work. The hydrogenation product of 3-methyl-1-pentene is 3-methylpentane, ΔHfo = -41.0 kcal/mol. Looking at the ΔHfo of typical 1-alkenes, one can see: 1-butene (ΔHfo = 0 kcal/mol), 1-pentene (ΔHfo = -5.0 kcal/mol) and 1-hexene (ΔHfo = 10.2 kcal/mol). Notice each one differs by ~5 kcal/mol. Their respective alkanes are: n-butane (ΔHfo = -30.0 kcal/mol), n-pentane (ΔHfo = -35.1 kcal/mol) and 1-hexene (ΔHfo = -39.9 kcal/mol). The heats of hydrogenation of each of these is,respectively, the difference in the heats of formation: -30.0 kcal/mol, -30.1 -30.0 kcal/mol and -30.0 kcal/mol-29.7 -30.0 kcal/mol. The average is -29.9 kcal/mol. Therefore, the ΔHfo of 3-methyl-1-pentene is x = -41.0 -(-29.9) = -11.1 kcal/mol. The reported ΔHfo of 2-methyl-1-butene is -6.1 kcal/mol. Add a methylene to this structure (-5.0 kcal/mol) and you obtain ΔHfo = -11.1 kcal/mol for 3-methyl-1-pentene. b) Calculate the heat of
hydrogenation of (E)-and
(Z)-3-methyl-2-pentene. Show work.
From the Heats of
Formation Table, (E)-3-methyl-2-pentene is ΔHfo
= -15.2 kcal/mol and (Z)-3-methyl-2-pentene is ΔHfo
= -14.8 kcal/mol. The heat of formation of 3-methylpentane
is ΔHfo = -41.0 kcal/mol. The
heat of formation of the (E)-isomer is -41.0 - (-15.2) =
-25.8 kcal/mol. The heat of formation of the (Z)-isomer is
-41.0 - (-14.8) = -26.2
kcal/mol. From the
diagram on the right, ΔHfo(E)
+ HHo(E) = ΔHfo(Z)
+ HHo(Z). Therefore, ΔHfo(E)
- Hfo(Z) = ΔHHo(Z)
- HHo(E). The (E)-isomer is
more stable. more negative heat of formation; lower
heat of hydrogenation. |
|
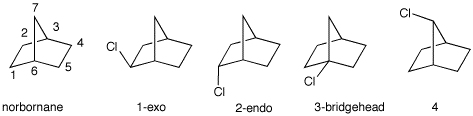 Positions 1, 2, 4 and 5 are all the same type of CH2 [8 x 4.5 = 36]. They lead to the same radical. Position 7 is a unique CH2 [2 x 4.5 = 9]. Positions 3 and 6 are bridgehead hydrogens [2 x 5.5 = 11]. Dividing each of these products by the sum (56) gives the relative amounts: 16%, 64% and 20%, respectively. b) The experimental result is: 1,
72%; 2, 25%; 4, 3%; 3, 0%. Why is no
bridgehead isomer formed even though tertiary C-H bonds are
highly reactive?
The radical from
the the bridgehead hydrogen cannot become planar. e) Are the four monochlorides optically
active, racemic, or achiral?
Exo and endo are
racemic; the other two are achiral. |
How to manipulate Jmol structures.
|
|
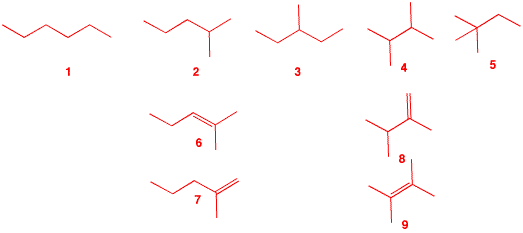
4. The major product B from free radical monobromination of alkane A (C6H14) readily reacts with water to form C, C6H14O. Treatment of bromide B with KOH in ethanol produces two, and only two, compounds, D and E. Compound D liberates 1.6 kcal/mol more heat upon hydrogenation to A than does E. What are the structures A-E? What is the heat of isomerization of D to E? Illustrate and explain. [Hint: What are the structures possible for A?] There are
five possible structures, 1-5,
for the acyclic, saturated alkane
A. Since bromide
B reacts readily with water
(SN1) to form alcohol
C, structures
1 and 5
are eliminated. Structure 3 is
also eliminated because E2 elimination of
3-bromo-3-methylpentane could give three alkenes:
2-ethyl-1-butene and (E)- and
(Z)-3-methyl-2-pentene. That leaves
6 and 7
from 2 and
8 and 9
from 4 for the structures of
D and E.
Lookup the heats of formation of
4, 8 and
9 in the the
Heats
of Formation
Tables.
From these data, one calculates the difference in
the heat of hydrogenation as 1.1 kcal/mol, which
isn't the value 1.6 kcal/mol. Alternatively, Table
7-1 in Wade gives the difference as 1.4 kcal/mol.
(there is some discrepancy here.) Neither
6 nor 7
appear in the heats of formation table but we can
look at lower homologs and calculate the heats of
formation using the 5.0 kcal/mol/CH2.
Thus, 2-methyl-1-butene (ΔHfo
= -8.4 kcal/mol) gives ΔHfo
= -13.4 kcal/mol for 7 while
2-methyl-2-butene (ΔHfo
= -10.0 kcal/mol) affords ΔHfo
= -15.0 kcal/mol for 6. The
difference is 1.6 kcal/mol. [There is actually
no need to include the 5 kcal/mol correction
because the difference would be the same.]
Using Table 7-1 in Wade, a value of 1.6 kcal/mol is
obtained. So, A =
2-methylpentane; B =
2-bromo-2-methylpentane; C =
2-methyl-2-pentanol; D =
2-methyl-1-pentene (7);
E = 2-methyl-2-pentene
(6). D,
with a less negative heat of formation than
E, liberates more heat on
hydrogenation. |
|
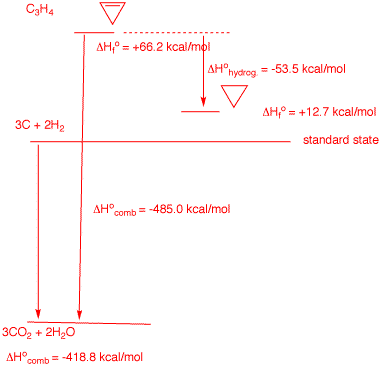
5. In 1968 Wiberg and Fenoglio determined the heat of combustion of cyclopropene: -485.0 kcal/mol. Determine the heat of hydrogenation of cyclopropene to cyclopropane. Explain and illustrate with a diagram. What is needed is the heat of formation of both cyclopropene and cyclopropane. The alkene's heat of formation is calculated as +66.2 kcal/mol in the usual way. See left side of diagram on the right. The heat of formation of cyclopropane comes from the Heats of Formation Tables. The difference in heats of formation is the heat of hydrogenation: -53.5 kcal/mol. In the chart below the heats of formation of simple cycloalkenes and cycloalkanes are listed. The heats of hydrogenation are computed. Cyclopropene's double bond is really distorted (bond angles). Note that the heat of hydrogenation of cyclohexene is no different from the heat of hydrogenation of (Z)-2-butene. 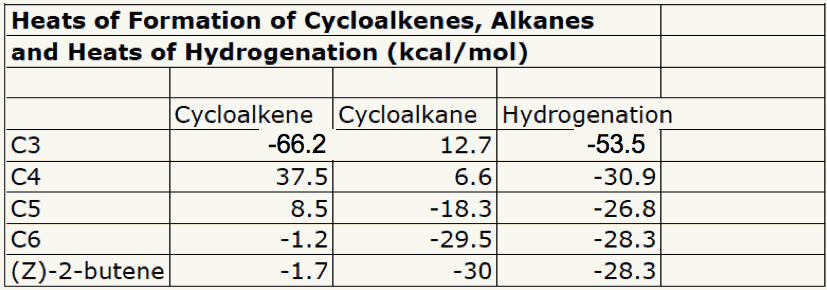 |
 |
|
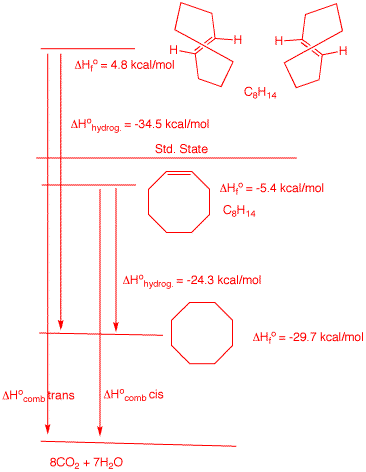
6. Two stereoisomers, A and
B, absorb one equivalent of hydrogen upon catalytic
hydrogenation to form cyclooctane. Compound A, which
is capable of resolution, liberates 34.5 kcal/mol of heat
while B liberates 24.3 kcal/mol of heat. |
 |