 |
|
(This photograph is in the hallway across from 110 SCL)
|
Reading and Enrichment Assignments:
Problem Set 2
Chapter 3, Alkanes
Due: Monday, September 20, 2010
(This photograph is in
the hallway across from 110 SCL) In 1885, as an addendum to a paper on
acetylenic compounds, Baeyer proposed that cyclopentane
was the least
strained of the cycloalkanes.
While he accepted the idea that the carbon atoms in
cycloalkanes were tetrahedral, he treated the
cycloalkanes as though they were flat. He argued that
there is only one cyclohexane carboxylic acid, not two
(axial and equatorial) as was predicted by a chair
cyclohexane.

Reading and Enrichment
Assignments:
a. Work through How to Draw Cyclohexanes (PowerPoint)
b. The Conformation Module in the Study Aids will give you a good overview of the subject of conformation.
c. View The Evolution of Formulas and Structure in Organic Chemistry During the 19th Century (PowerPoint).
1. Redraw (line angle formula) and name (IUPAC) the hydrocarbon in this problem. For a dynamic view click here. For a static view click here. How to manipulate Jmol structures. [What if there are two different longest chains? Check here.]
2. Compound A (MW=162.61), a 1,4-disubstituted cyclohexane,
has the following composition: C, 51.70%; H, 6.82%; Cl, 21.80%. The
difference in conformational energy for the two chair conformations
of A is 1.9 kcal/mol. Using the A-value
data (Energy Differences Between ..... Cyclohexanes), determine the
structure of A. Illustrate and explain. What is the
conformational energy difference for the stereoisomer of A,
---namely A'. Explain and illustrate. Show the chair
comformations of A and A' with the appropriate
equilibrium arrows to illustrate the major and minor conformations.
Label each conformation with its energy.
The first order of business is to determine the molecular formula of the compound. Does compound A contain only C, H and N? No! The sum of the percentages adds up to 80.32%. Since oxygen is determined by difference it must constitute 19.68% of the remaining matter. For the calculation:
The formula is C7H11ClO2. M.W.
calculated: 162.61, which agrees with the given value. [Note: A
compound composed of these four elements must, if it has an odd
number of halogens,, must have an odd number of hydrogens. Thus, the
number 11.42 in the chart must be rounded down and NOT up.
Check
here.] Compound
A is a 1,4-disubstituted cyclohexane.
Cyclohexane is C6H12. Subtracting two hydrogens
for the positions of the two substituents leaves a cyclohexane
nucleus of C6H10. Subtracting:
C7H11ClO2 -
C6H10 = CHClO2 for the sum of the
composition of the two substituents. Although there are several
permutations for these groups, There is only one chlorine containing
group --- chlorine (0.5 kcal/mol) itself. Therefore, the other group
must be CO2H (1.4 kcal/mol). Since the energy difference
between the two chair conformations of A is the sum of the two
groups, both groups must be axial (1.9 kcal/mol) in one conformation
and diequatorial (0 kcal/mol) in the other conformation. A is
trans-4-chlorocyclohexanecarboxylic acid. Stereoisomer A' is the cis
isomer whose energy difference between the two chair conformations is
1.4 - 0.5 = 0.9 kcal/mol. See below:
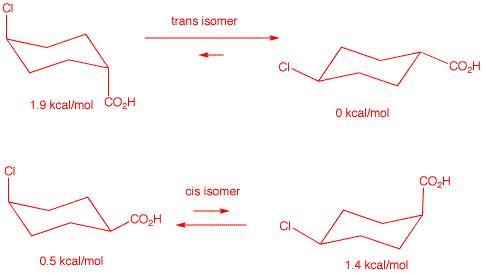 :
:
3. Predict the heat of formation of 2-methyloctane using the data
presented here.
Explain.
There are several 2-substituted alkanes listed: 2-methylbutane (-36.7 kcal/mol), 2-methylpentane (-41.7 kcal/mol), 2-methylhexane
(-46.6 kcal/mol) and
2-methylheptane (-51.1 kcal/mol). This series adds one
-CH2- group at an average value of -5
kcal/mol/CH2. Thus, 2-methyloctane's heat of combustion
can be estimated as -51.5 -5 = -56.5 kcal/mol.
4. Examine the heats of formation of the four octanes listed in the
heats
of formation tables.
a)What trend do you notice?
b) Draw a diagram that shows the heat of formation and heat of combustion of the two extreme cases: n-octane and 2,2,3,3-tetramethylbutane. Show calculations.
c) Using the Jmol structures located here, measure the C-H, CH3-CH2 and CH2-CH2 bond lengths for n-octane. Measure the three different bond lengths for 2,2,3,3-tetramethylbutane. Record all six values. What trend do you see?
d) If bond breaking is critical to combustion, how do the results in 4c account for the heats of formation of these two octanes?
.
5. Calculate the heat of combustion of cyclobutane using the
data (ΔHfo of cyclobutane,
CO2 and H2O) in the heats
of formation tables.
Compare your value with
the value in Table 3-5 in your text.
6. Draw Newman projections for the eclipsed and staggered
conformations of 2-methylbutane viewed along the
C2-C3 axis. Calculate the energy of each
conformation, both staggered and eclipsed.