Chem 220 - Organic Chemistry
Solution Set
Problem Set 2
Chapter 3, Alkanes
Due: Monday, September 20,
2010

The Baeyer Laboratory,
Munich, 1893
(This photograph is in
the hallway across from 110 SCL)
Adolf
von Baeyer (1835-1917); Nobel
Prize 1905. (center, seated with derby), who was a student
of Kekulé, succeeded Liebig at Munich. In the
photograph (second row; third from right) is
Henry Lord
Wheeler (1867-1914); Yale Faculty
1896-1911. As was the custom in the 19th century,
many Americans, such as Wheeler, did advanced study in
chemistry in Europe.
Karl
is the laboratory assistant. (The only person wearing an
apron and no tie; upper left.)
In 1885, as an addendum to a paper on
acetylenic compounds, Baeyer proposed that cyclopentane was
the least
strained of the cycloalkanes.
While he accepted the idea that the carbon atoms in
cycloalkanes were tetrahedral, he treated the cycloalkanes
as though they were flat. He argued that there is only one
cyclohexane carboxylic acid, not two (axial and equatorial)
as was predicted by a chair cyclohexane.
|

Sir Derek H. R. Barton
(1918-1998)
1969 Nobel
Prize with Odd Hassel for their
work on conformational analysis
For a video of Barton talking about
conformational analysis, click
here.
Cyclohexane
in the chair conformation
(How
to manipulate Jmol
structures)
|
Reading and Enrichment
Assignments:
a. Work through How
to Draw Cyclohexanes (PowerPoint)
b. The Conformation
Module in the Study Aids will give you a
good overview of the subject of conformation.
c. View The
Evolution of Formulas and Structure in Organic Chemistry During the
19th Century (PowerPoint).
1. Redraw (line angle formula) and name (IUPAC)
the hydrocarbon in this problem. For a dynamic view click
here.
For a static view click here.
How
to manipulate Jmol structures. [What
if there are two different longest chains? Check
here.]
4-Isopropyl-2,6-dimethyloctane
2. Compound A (MW=162.61), a 1,4-disubstituted cyclohexane,
has the following composition: C, 51.70%; H, 6.82%; Cl, 21.80%. The
difference in conformational energy for the two chair conformations
of A is 1.9 kcal/mol. Using the A-value
data (Energy Differences Between ..... Cyclohexanes), determine the
structure of A. Illustrate and explain. What is the
conformational energy difference for the stereoisomer of A,
---namely A'. Explain and illustrate. Show the chair
comformations of A and A' with the appropriate
equilibrium arrows to illustrate the major and minor conformations.
Label each conformation with its energy.
The first order of business is
to determine the molecular formula of the compound. Does compound A
contain only C, H and N? No! The sum of the percentages adds up to
80.32%. Since oxygen is determined by difference it must constitute
19.68% of the remaining matter. For the calculation:
|
Atom
|
At.
Wt.
|
%/At.
Wt.
|
%/At.
Wt./0.61
|
Rounding
|
|
C
|
12.01
|
4.30
|
7.05
|
7
|
|
H
|
1.008
|
6.76
|
11.08
|
11
|
|
Cl
|
35.45
|
0.61
|
1
|
1
|
|
O
|
16
|
1.23
|
2.02
|
2
|
The formula is C7H11ClO2. M.W.
calculated: 162.61, which agrees with the given value. [Note: A
compound composed of these four elements must, if it has an odd
number of halogens,, must have an odd number of hydrogens.
Check
here.] Compound
A is a 1,4-disubstituted cyclohexane.
Cyclohexane is C6H12. Subtracting two hydrogens
for the positions of the two substituents leaves a cyclohexane
nucleus of C6H10. Subtracting:
C7H11ClO2 -
C6H10 = CHClO2 for the sum of the
composition of the two substituents. Although there are several
permutations for these groups, there is only one chlorine containing
group --- chlorine (0.5 kcal/mol) itself. Therefore, the other group
must be CO2H (1.4 kcal/mol). Since the energy difference
between the two chair conformations of A is the sum of the two
groups, both groups must be axial (1.9 kcal/mol) in one conformation
and diequatorial (0 kcal/mol) in the other conformation. A is
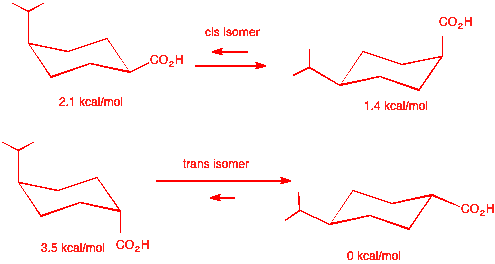
trans-4-chlorocyclohexanecarboxylic acid. Stereoisomer A' is the cis
isomer whose energy difference between the two chair conformations is
1.4 - 0.5 = 0.9 kcal/mol. See below:
 :
:
3. Predict the heat of formation of 2-methyloctane using the data
presented here.
Explain.
There are several 2-substituted
alkanes listed: 2-methylbutane (-36.7 kcal/mol), 2-methylpentane
(-41.7 kcal/mol), 2-methylhexane
(-46.6 kcal/mol) and
2-methylheptane (-51.1 kcal/mol). This series adds one
-CH2- group at an average value of -5
kcal/mol/CH2. Thus, 2-methyloctane's heat of combustion
can be estimated as -51.5 -5 = -56.5 kcal/mol.
4. Examine the heats of formation of the four octanes listed in the
heats
of formation
tables.
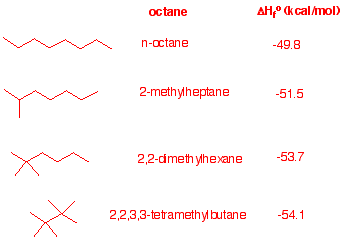
a)What trend do you notice?
Branching gives a more
negative heat of formation.
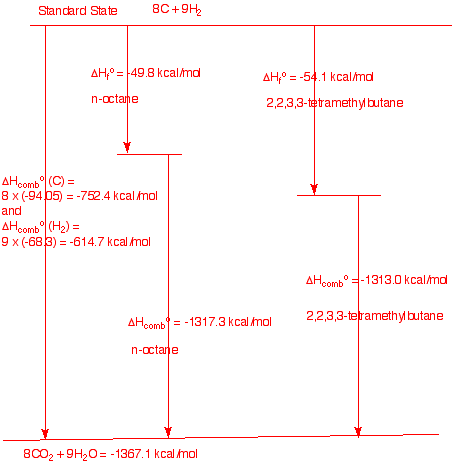
b) Draw a diagram that shows the
heat of formation and heat of combustion of the two extreme cases:
n-octane and 2,2,3,3-tetramethylbutane.
Show calculations. In
ractice the heats of formation are determined by combusting the
appropriate amounts of graphite and hydrogen to et the heat of
combustions from the the elements (ΔHfo =
0 kcal/mole). Combustion of the compounds gives a value whose
difference from the elements determines the heat of formation. In
this instance we are calculating the heat of combustion of two
isomers knowing the heats of formation. clearly, the difference in
the heat of combustion for a compound equals the difference in the
heat of formation.(Chart not to scale.)
.
5. a) Calculate the heat of combustion of cyclobutane using the
data (ΔHfo of cyclobutane,
CO2 and H2O) in the heats
of formation tables.
Compare your value with
the value in Table 3-5 in your text.
Cyclobutane has the formula
C4H8 (ΔHfo = +6.6
kcal/mol). The heat of combustion of four moles of graphite and four
moles of hydrogen is 4 x [(-94.05) + (-68.3)] = -649.4
kcal/mol. The heat of combustion of cyclobutane = -649.4 - (+6.6) =
-656 kcal/mol. compares well with the table. Note that cyclobutane is
less stable than the atoms from which it is formed.
b) Calculate the strain energy in cyclobutane given the heat of
combustion of cyclohexane (Table 3-5 in your text) and the knowledge
that cyclohexane is strain-free.
Cyclobutane has 2/3 as many
methylene groups as cyclohexane. Therefore, its heat of combustion
should be 2/3 the heat of combustion of cyclohexane or 2/3 x (-944.4)
= -629.6 kcal/mol for strain-free cyclobutane. But cyclobutane has a
heat of combustion of -655.8 kcal/mol. The difference between these
numbers is the strain energy: 26.2 kcal/mol.
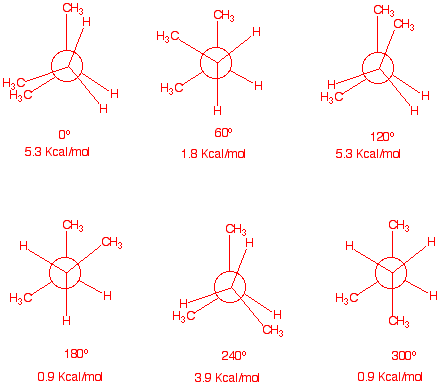
6. Draw Newman projections for the
eclipsed and staggered conformations of 2-methylbutane viewed along
the C2-C3 axis. Calculate the energy of each
conformation, both staggered and eclipsed.
It is irrelevant which
eclipsed conformation is taken as 0o.


 :
:

