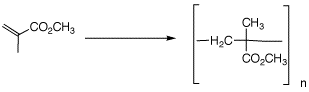
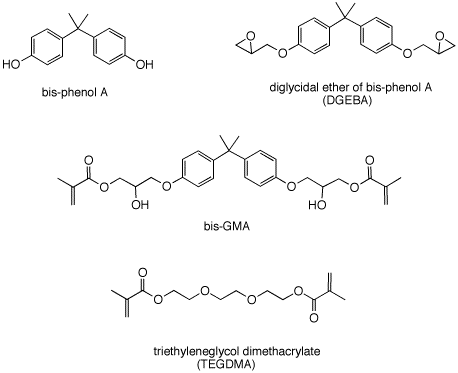
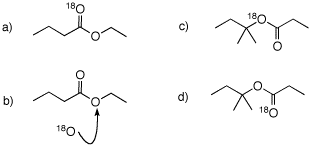
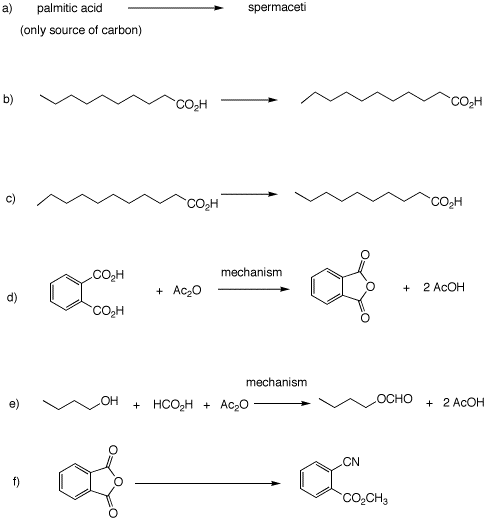
Problem Set 7
Chapters 20 & 21
Carboxylic Acids and Their Derivatives
Due: Monday, March 28, 2005
Esters are pleasant smelling, volatile
compounds. Isoamyl acetate (oil of banana), ethyl butyrate
(pineapple), and methyl salicylate (wintergreen) to name a
few. Apparently esters are pleasant to roaches. The February 18 issue of Science (2005,
pg. 1104) reveals how entomologists
and chemists unraveled the
structure of the sex pheromone of the ubiquitous German
cockroach, Blattella germanica. HTML
version. PDF
version. Blattellaquinone 1. Read the short paper cited above and
see what issues apply to what you have learned in organic
chemistry. The PDF version is better for general reading but
the HTML permits blow-ups of the nmr spectrum if you need to
measure coupling constants. a) What was the source of the
pheromone? Jfg = Jef = 8.6 Hz
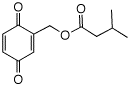
b) How was blattellaquinone detected by gas
chromatography?
c) Account for the peak m/z = 180 in the EI mass
spectrum.
d) Given the 1H NMR data on the right, assign
each of the hydrogens (Ha-g) to a chemical shift
and explain the multiplicity and coupling patterns.
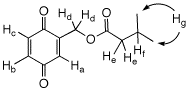
Jab = Jad = 2.0 Hz
Jbc = 10.0 Hz